Accelerate Your Cardiovascular Device Production
End-to-End Assembly, Kitting, Packaging, and Sterilization Services
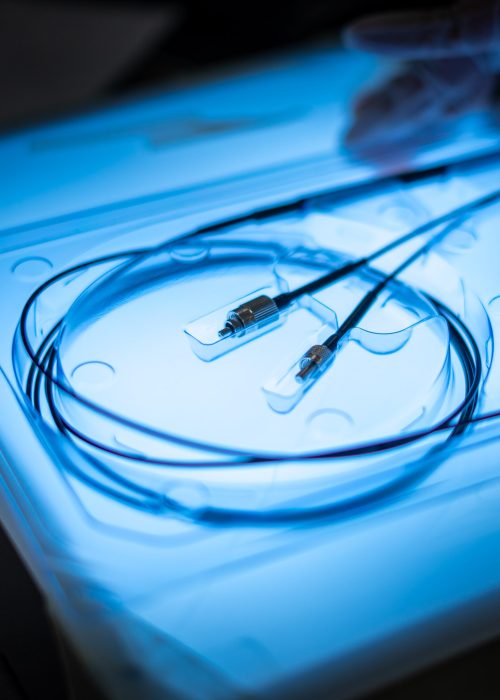
Cardiovascular Device Expertise
Cardiovascular device manufacturers operate in one of the most highly regulated and rapidly evolving medical fields. From stringent FDA and ISO requirements to the need for precise assembly, sterilization, and packaging, navigating the complexities of cardiovascular device production requires an experienced partner.
For over 2 decades, LSO has helped leading and emerging cardiovascular OEMs accelerate time-to-market with scalable, compliant, and cost-effective manufacturing solutions—ensuring quality without compromise.
Cardiovascular Device Capabilities
Life Science Outsourcing has extensive experience supporting the production, packaging, and distribution of cardiovascular medical devices.


Cleaning & Decontamination
We provide precision cleaning and decontamination services to eliminate biohazards and ensure compliance with critical safety and sterility standards.
Cleanroom Assembly & Kitting
From intricate catheter assemblies to complete procedural kits, LSO delivers turnkey cleanroom manufacturing and kitting solutions tailored to cardiovascular devices.


Labeling
Our in-house labeling expertise ensures compliance with FDA, ISO, and UDI (Unique Device Identification) regulations while maintaining the highest quality standards.
Sterilization
LSO offers comprehensive contract sterilization services, including validation and documentation support, to meet the stringent requirements for single-use and reusable cardiovascular devices.


Product Portfolio
- Catheters (diagnostic, interventional, and ablation)
- Stents and vascular grafts
- Guidewires
- Embolic protection devices
- Balloon angioplasty devices
- Heart valve implants
- Cardiac monitoring and electrophysiology devices
Why LSO?
Specialized Expertise for Cardiovascular Device OEMs
Cardiovascular devices demand the highest levels of precision, quality, and compliance. LSO’s integrated manufacturing and sterilization solutions help streamline production while ensuring regulatory success.
- Faster Time-to-Market
Accelerate product launches with seamless cleanroom assembly, packaging, and sterilization services that reduce regulatory bottlenecks. - Regulatory & Compliance Mastery
Ensure compliance with FDA, ISO 13485, and global regulatory standards with our in-depth expertise and quality-driven processes. - Cost-Effective, Scalable Solutions
Minimize costs and production risks with fully integrated in-house services designed to support everything from clinical trials to full-scale commercialization. - End-to-End Manufacturing & Sterilization Expertise
From catheter assembly and kitting to contract sterilization and regulatory documentation, we provide a one-stop solution for cardiovascular medical device production.
