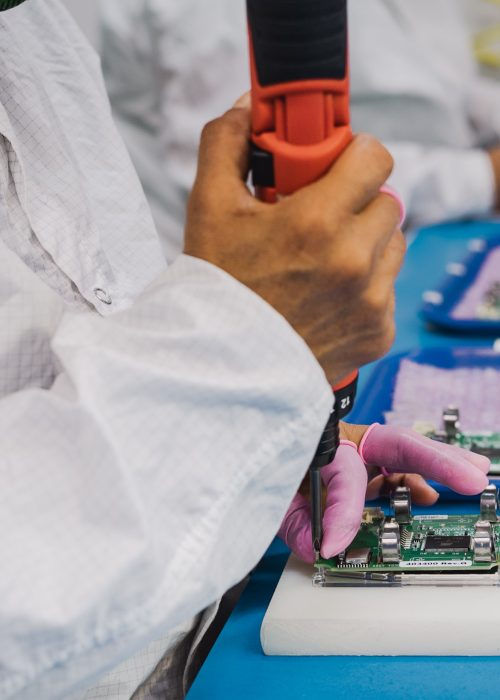
Life Science Outsourcing specializes in complex medical device assembly, ensuring high-precision component integration, cleanroom-controlled processes, and compliance with industry regulations.

LSO specializes in high-precision medical device assembly, integrating complex components in ISO-certified cleanrooms to ensure quality and compliance. Our validated processes help OEMs, orthopedic manufacturers, and electromechanical device companies scale efficiently and accelerate time to market. Scroll down to see how we can support your assembly needs.
Complex assembly involves the precise integration of multiple components into a finished medical device.





Comprehensive packages for regulatory compliance.

From fractional to full cycles with EtO residual testing.

Secure with 2X qualification.

Tailored to your sterilization needs.
Receive the latest infographics, guides, and blog updates for medical device manufacturing, package testing, and sterilization.