Small-batch, quick-turn, in-house EtO sterilization delivers rapid processing in as little as 5-7 days (24-hour expedited option available) without compromising quality or compliance.

LSO operates 3M™ sterilizers on both the East and West Coasts, ensuring fast and compliant sterilization services. These small-batch, canister-based sterilizers support 1-8 hour cycles with in-chamber pre-conditioning, sterilization, and aeration. They maintain temperature ranges of 37-55°C while meeting ISO 11135, EPA, OSHA, SCAQMD, and AAMI standards.
Unlike large-scale sterilization facilities facing regulatory challenges, our approach reduces environmental impact and ensures uninterrupted service.
Small-batch canister-based EtO sterilization reduces exposure risks and environmental impact while retaining the efficacy and compatibility that make EtO the industry’s gold standard.
Ethylene Oxide (EtO) sterilization leverages a colorless gas that functions at relatively low temperatures. This approach currently stands out as the predominant sterilization technique for medical devices. Products are enclosed within a secure chamber where they are exposed to EtO gas. EtO inactivates microorganisms through alkylation of their molecular structures and genetic material. EtO has excellent compatibility with an array of materials, ranging from plastics and metals to glass.

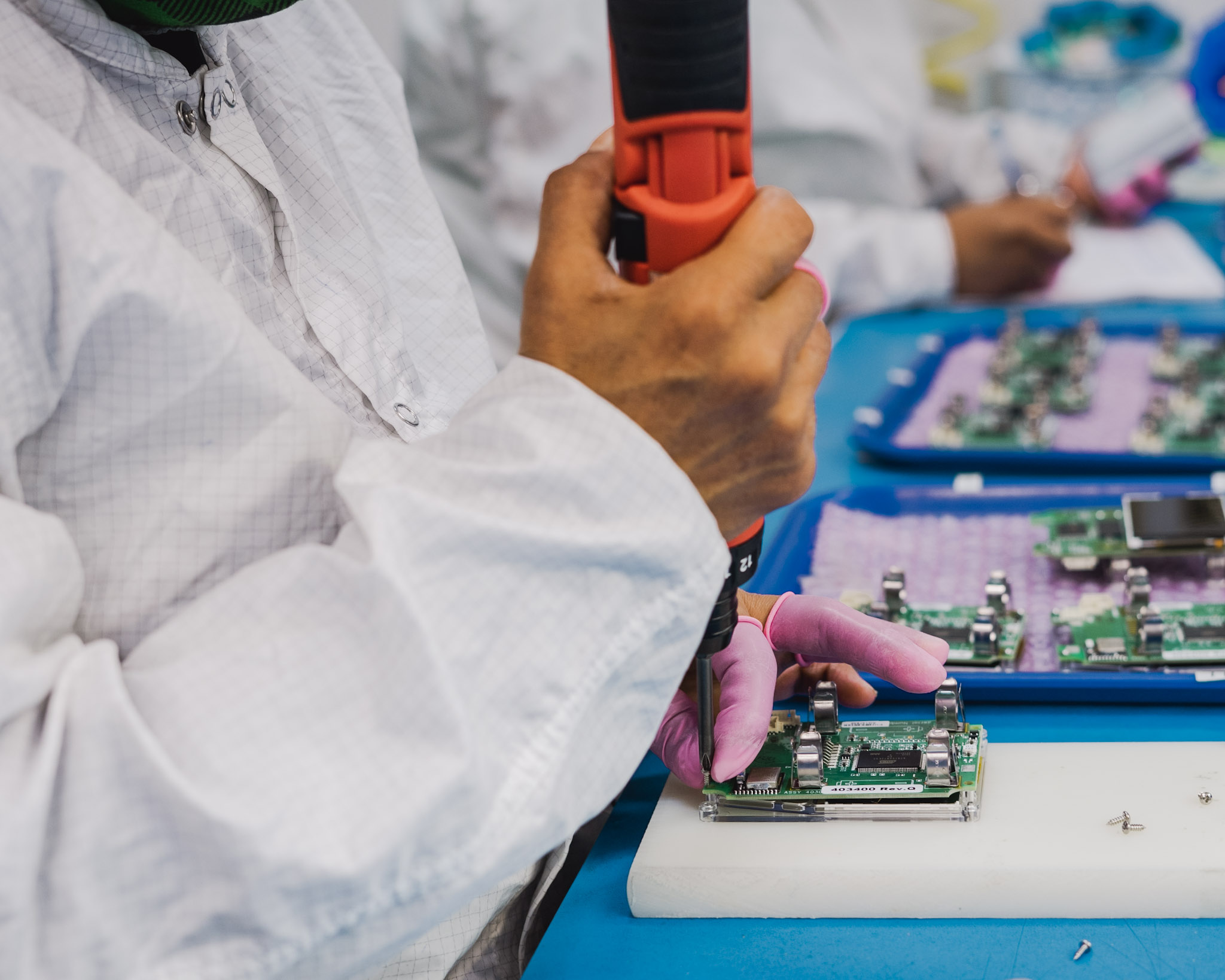
Ethylene oxide sterilization is particularly effective for devices that are unable to tolerate high heat and radiation. It is widely used for pacemakers, delicate implants, surgical kits, syringes, and catheters. This method is beneficial for sterilizing a broad range of materials including plastic resins, metals, glass, and multi-layer packaging. As a surface sterilant, EtO is unable to travel past hermetic seals and gas-tight fittings.
At LSO, we utilize six (4 LSO West, 2 LSO East) in-house 3M™ In-House sterilizers with ‘All-In-One’ cycles. During the 1-8 hour (4 hour standard) customizable exposure times, pre-conditioning, sterilization, and aeration are performed in-chamber. Temperatures can range from 37-55 degrees C to suit different material requirements while meeting ISO 11135-2014 standards and conforming to all regulatory requirements (EPA OSHA, SCAQMD, AAMI) for quality and safety.

Our EtO Capacity Calculator helps you determine exactly how many cartons will fit in a standard sterilization basket based on your product dimensions.
Simply enter your carton measurements and instantly receive optimized loading configurations.
LSO maintains multiple ethylene oxide sterilizers across our California and New Hampshire facilities, providing significant capacity to meet your medical device sterilization needs.
With customizable cycle parameters and temperatures ranging from 37-55°C, we accommodate diverse material requirements while meeting ISO 11135-2014 standards.
Contact our team today to discuss your sterilization requirements – we have the capacity and expertise ready to support your product needs.
In addition to our ‘always open’ capacity, we offer professional expertise with our in-house microbiologist, maintain the highest levels of compliance and certification, and have successfully assisted over 1,000 clients in processing numerous sterilization cycles and validations. With facilities on both coasts, we provide unparalleled access and support, ensuring faster turnaround times and flexibility for clients across the country. Building on this extensive experience, we streamline your sterilization process for ease and efficiency with:
We are committed to delivering end-to-end medical device sterilization and packaging support to our clients.

Comprehensive packages for regulatory compliance.

From fractional to full cycles with EtO residual testing.

Secure with 2X qualification.

Tailored to your sterilization needs.
Life Science Outsourcing is an FDA registered and ISO 13485 certified full service Medical Device Contract Manufacturer.
We improve our products and advertising by using Microsoft Clarity to see how you use our website. By using our site, you agree that we and Microsoft can collect and use this data. Our privacy notice has more details.
Receive the latest infographics, guides, and blog updates for medical device manufacturing, package testing, and sterilization.