Accelerate your product launch with FDA-registered, ISO 13485-certified manufacturing. From cleanroom assembly to packaging, labeling, and sterilization—LSO delivers compliant, turnkey solutions for medical devices and implants.

Bringing life-saving medical devices and implants to market requires precision, compliance, and efficiency. At Life Science Outsourcing (LSO), we offer comprehensive contract manufacturing services designed to streamline production, ensure regulatory compliance, and accelerate commercialization for medical device manufacturers.
As an FDA-registered, ISO 13485-certified contract manufacturer, we specialize in assembly, packaging, sterilization, labeling, and supply chain management—all under one roof. Whether you’re developing orthopedic implants, cardiovascular devices, or minimally invasive surgical tools, our scalable, flexible solutions help bring your products to market faster and more efficiently.
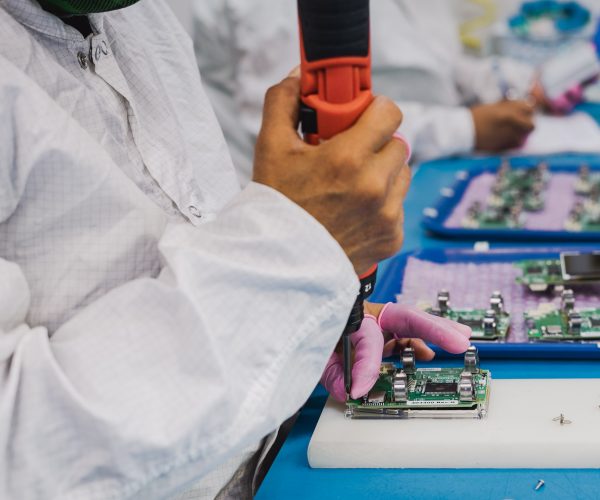
From simple subassemblies to complex medical implants, our cleanroom assembly services meet the highest quality and regulatory standards.
We offer:


Ensure your devices are sterile, compliant, and market-ready with our custom packaging and kitting solutions. We specialize in:
Our complex assembly services are designed for medical devices that require:

Regulatory-compliant labeling is critical for traceability, UDI compliance, and patient safety. LSO offers:
We help you reduce risk, lower costs, and optimize supply chain efficiency with:


Ensure biocompatibility and long-term device integrity with our:
Receive the latest infographics, guides, and blog updates for medical device manufacturing, package testing, and sterilization.