Microbial identification testing for sterilized medical devices
Microbial identification tests identify and characterize microorganisms before or after sterilization.

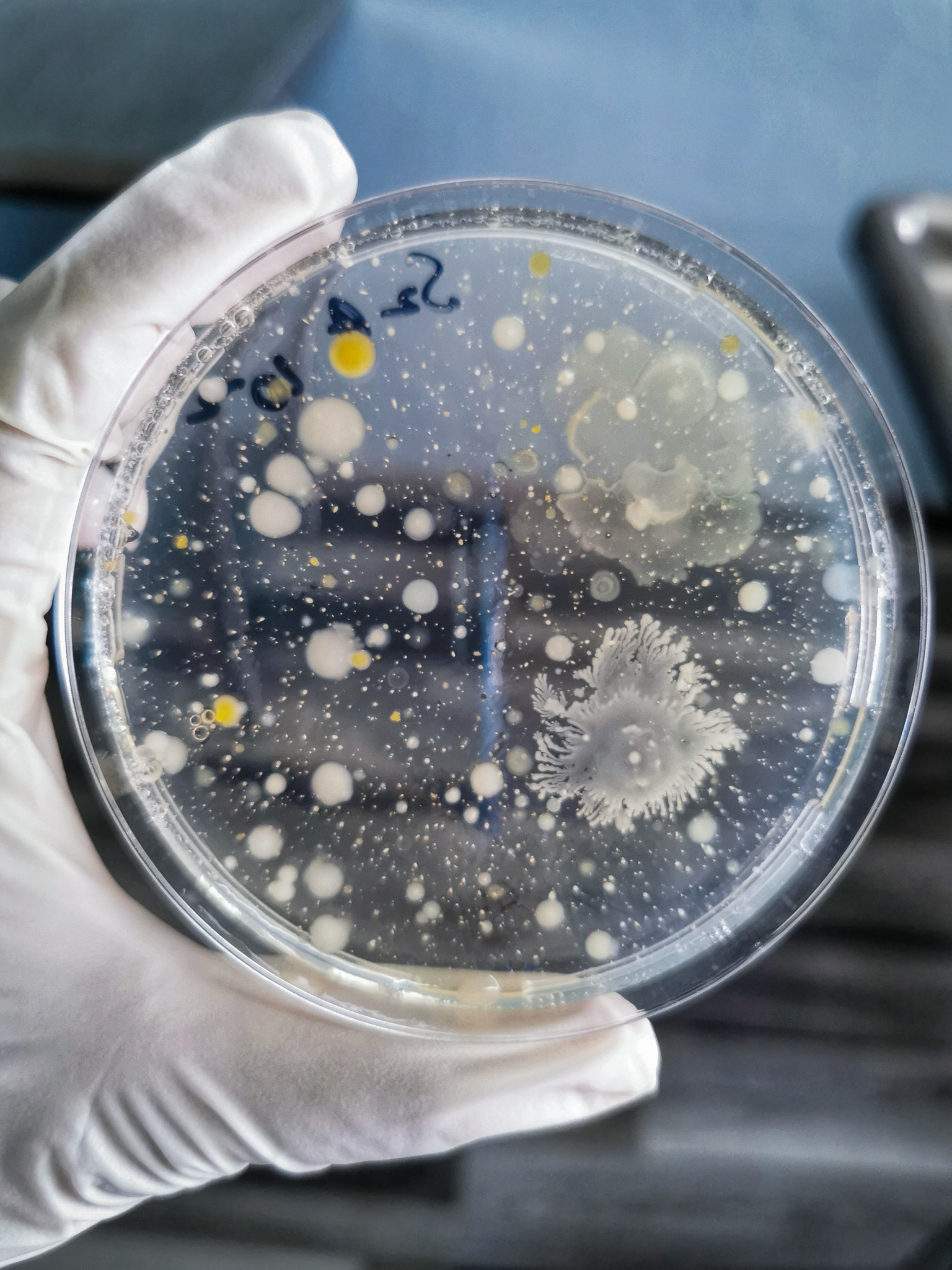
What is microbial identification testing?
During sterilization validation and routine monitoring, microbial identification testing occurs to characterize natural product bioburden. Identification can also be performed after the sterilization process to investigate biological indicator sterility failures. The test can include various methods, including biochemical tests, molecular techniques, and phenotypic analysis.

Who needs microbial identifications testing?
Medical device manufacturers and OEMs involved in producing and supplying medical devices conduct these tests as needed to meet regulatory compliance and ensure product safety.
How does microbial identifications testing work?
This type of testing has several steps:
Sample collection
Sample collection can be done by swabbing, rinsing, or direct contact.
Culture methods
Samples are incubated in a controlled environment. The growth of any colonies or turbidity is then observed.
Isolation of pure cultures
Individual colonies or liquid dilutions are transferred to fresh media to obtain pure cultures.
Identification techniques
Biochemical tests can determine microorganism phenotypes such as enzymatic activities. Molecular techniques include polymerase chain reaction (PCR) and DNA sequencing.MALDI-TOF mass spectrometry may also be used. Automated systems can rapidly identify microorganisms using reference databases.
Data interpretation and reporting
The obtained data is compared with established microbial databases to identify microorganisms, and detailed reports are created.
Verification and validation
Positive and negative controls are used throughout the process for accuracy and reliability.


What standards apply?
The FDA has outlined regulatory requirements in the United States for medical devices. Microbial identifications testing is an important component to meet such requirements.
ISO 11737-1 and specify requirements for determining bioburden on medical devices and during sterilization validation.
Why LSO?
As an FDA-registered and ISO 13485-certified contract manufacturing organization, Life Science Outsourcing (LSO) offers assembly, packaging, sterilization, and specialized capabilities in diagnostics packaging and design. Our unique business model combines comprehensive in-house services and extensive regulatory expertise, allowing us to provide the agility and flexibility needed to expedite market launches while standardizing the supply chain and minimizing risks.
