Peel (Seal) Strength Testing
Takes about 90 seconds.
Typical response within 1 business day.
15-minute consult with a packaging specialist.
Peel Strength (Seal Strength) Testing for Medical Device Packaging
Peel strength testing—also commonly referred to as seal strength testing—measures the force required to separate a package seal. This test provides a quantitative assessment of seal integrity and consistency for flexible, semi-rigid, and even rigid sterile barrier systems.
Peel and seal strength are frequently used interchangeably within medical packaging and regulatory documentation, particularly when referencing ASTM F88 or EN 868-5 testing.
Video Overview: Peel Strength (Seal Strength) Testing
What Peel Strength (Seal Strength) Testing Evaluates

Peel strength testing evaluates:
- Seal integrity
- Seal consistency across samples
- Effects of sealing parameters
- Seal performance after aging or distribution testing
Q: Is peel strength the same as seal strength?
A: Yes. In medical packaging, peel strength and seal strength are often used interchangeably to describe the force required to separate a package seal.
How Peel Strength Testing Works
Peel strength testing is commonly performed in accordance with ASTM F88 or EN 868-5. General steps include:
- Conditioning samples as required
- Cutting samples from the package.
- Clamping each side of the cut sample
- Pulling the seal apart at a controlled rate
- Recording the force required to separate the seal
- Evaluating results against acceptance criteria
- Interpret the results using average force and standard deviation of average force.
Q: Is peel strength testing destructive?
A: Yes. Peel strength testing is destructive because the package is cut open and the seal is separated during testing.

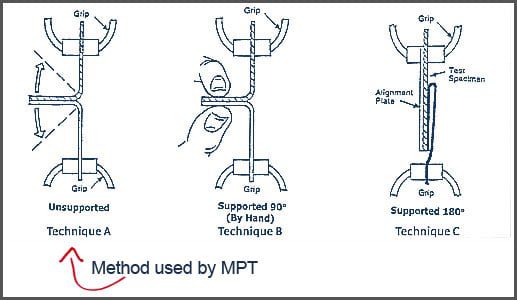
ASTM F88 Peel Test Methods
ASTM F88 defines multiple peel test techniques, including:
- 180-degree peel
- 90-degree peel (supported)
- 90-degree peel (unsupported)
Q: Does ASTM F88 specify which peel method to use?
A: ASTM F88 describes multiple methods, but the appropriate method must be justified based on the packaging configuration and test intent.
Packaging Types Commonly Tested
Peel (seal) strength testing is commonly applied to:
- Tyvek® pouches
- Film-to-film seals
- Paper-to-film packaging
- Form-fill-seal pouches
- Flexible sterile barrier systems
- Thermoformed trays with a Tyvek® lid
Q: Can rigid packaging be tested using peel strength methods?
A: Yes, it can but must be taken into account for the setup of the test and ability to produce a repeatable peel process.Peel Strength Data and Interpretation
Packaging Types Commonly Tested
Peel (seal) strength testing is commonly applied to:
- Tyvek® pouches
- Film-to-film seals
- Paper-to-film packaging
- Form-fill-seal pouches
- Flexible sterile barrier systems
- Thermoformed trays with a Tyvek® lid
Q: Can rigid packaging be tested using peel strength methods?
A: Yes, it can but must be taken into account for the setup of the test and ability to produce a repeatable peel process.Peel Strength Data and Interpretation
Peel strength results help evaluate:
- Seal uniformity
- Seal failure modes
- Process variability
- Effects of aging or distribution stress
Q: Is higher peel strength always better?
A: No. Peel strength must fall within an acceptable range to ensure both seal integrity and package usability. This range is determined by the test’s customer since ASTM F88 does not specify. If using EN 868-5, minimum values are recommended based on the type of sterilization used in the post-sealing process.
Role in Packaging Validation
Peel strength (seal strength) testing is commonly used as part of:
- Process validation
- Packaging validation per ISO 11607
- Stability and shelf-life studies
- Distribution testing programs
- Heat seal qualification
Q: Does peel strength testing support ISO 11607?
A: Yes. Peel strength testing is widely used to support seal integrity and packaging validation requirements under ISO 11607.
Peel Strength (Seal Strength) Testing FAQ
What is peel strength testing?
A test that measures the force required to separate a package seal.
Is peel strength the same as seal strength?
Yes. The terms are often used interchangeably in medical packaging.
Which standard governs peel strength testing?
ASTM F88 is the primary standard.
Is the test destructive?
Yes.
Takes about 90 seconds.
Typical response within 1 business day.
15-minute consult with a packaging specialist.

