Life Science Outsourcing has proven expertise in helping medical device manufacturers navigate the decontamination process, enhance efficiencies, and meet all regulatory requirements.
Streamline your reusable medical device processing with expert cleaning, decontamination, and sterilization. Ensure compliance with regulatory standards, reduce downtime, and maximize the value of your inventory. Safeguard patient safety while improving efficiency and responsiveness to your team’s needs.
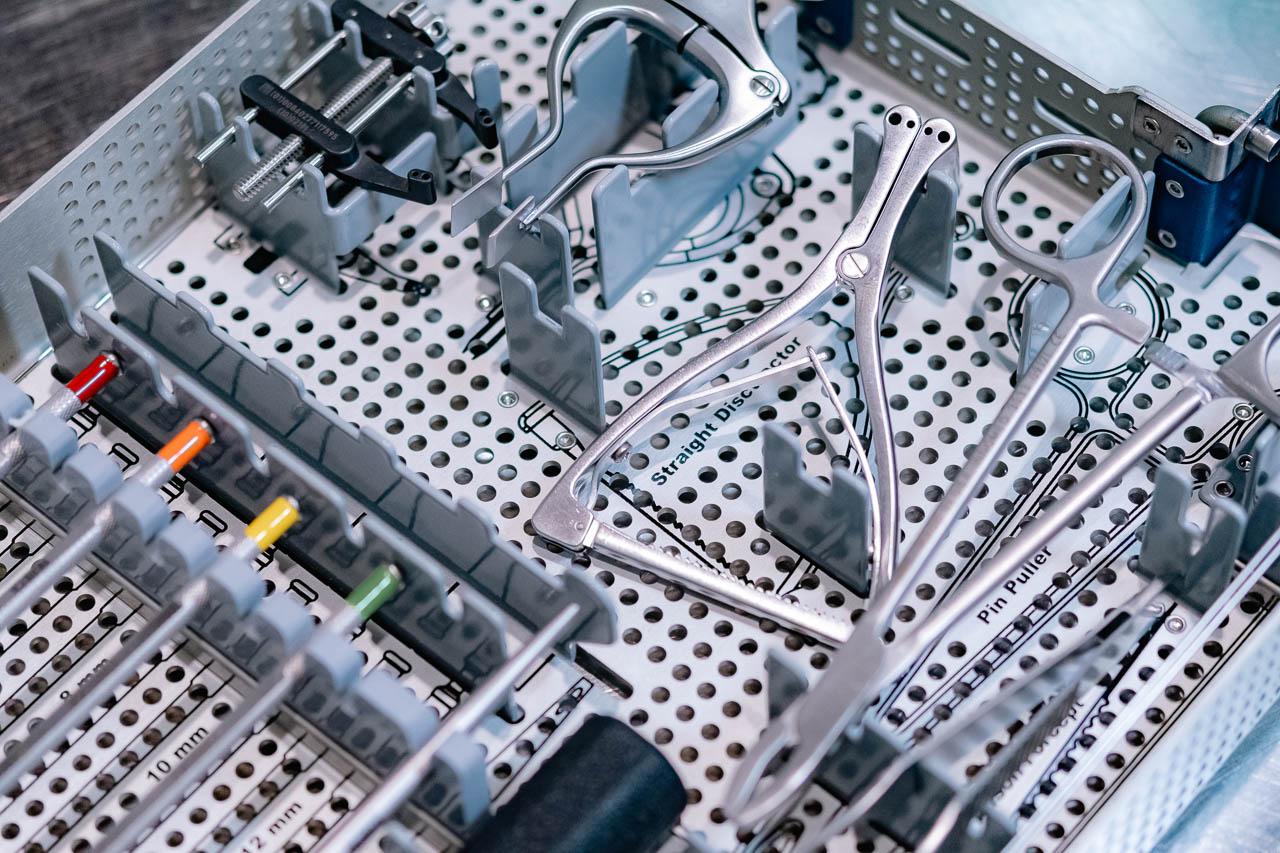
Reusable medical devices must be cleaned, disinfected, and sterilized following strict industry guidelines to ensure they are safe for reuse. These processes remove contaminants, eliminate pathogens, and meet regulatory requirements for medical device reprocessing.


Reusable medical device processing ensures orthopedic devices, surgical instruments, and delivery systems are properly decontaminated for safe reuse. This process removes pathogens, prevents infections, and meets strict regulatory standards.
Companies looking to validate single-use devices for reuse can benefit from expert decontamination services that ensure compliance, safety, and efficiency. By partnering with an experienced provider, organizations can extend product life, reduce waste, and optimize inventory management while maintaining regulatory compliance.
Hospitals and medical sales teams often face challenges with excess inventory of orthopedic devices. Without proper decontamination, returned products can’t be safely redeployed, leading to unnecessary waste and higher costs.
Life Science Outsourcing streamlines this process with comprehensive decontamination, inspection, and repackaging services. Our autoclave sterilization and quality-controlled methods ensure compliance, safety, and fast turnaround times.
By partnering with LSO, companies can reduce waste, cut costs, and efficiently manage returned inventory while maintaining compliance and quality.
Reusable medical devices undergo a structured decontamination process in three key steps, each increasing in intensity based on contamination levels.
The first and most crucial step in decontamination, cleaning removes both visible and non-visible contaminants (e.g., blood, protein substances, and debris) from the device surface using detergent and water. LSO typically uses Ecozyme for cleaning.
Liquid disinfectants eliminate non-spore-forming bacteria. Common disinfectants include:
This final step destroys microorganisms and eliminates transmissible agents like spores and bacteria. Life Science Outsourcing offers sterilization methods such as:

In addition to ISO 13485 (Quality Management System Guidelines), LSO adheres to other relevant sterilization standards including:
Sterility assurance level is defined as “the probability of a single unit being non-sterile after it has been subjected to the sterilization process.”
Once a device has been sterilized, tests are conducted to determine if the desired sterility levels have been obtained. LSO tests for this using both biological indicator (BI) testing and chemical indicators.
It is critical that prescribed guidelines are followed when packaging and transporting contaminated medical devices due to the biohazardous risk involved. Logistics companies and transportation organizations insist that packaging of diagnostic specimens and environmental test samples includes four basic components:
Life Science Outsourcing has proven expertise in helping medical device manufacturers navigate the decontamination process, enhance efficiencies, and meet all regulatory requirements.
Life Science Outsourcing is one of the few regulated services companies to offer EtO.

Comprehensive packages for regulatory compliance.

From fractional to full cycles with EtO residual testing.

Secure with 2X qualification.

Tailored to your sterilization needs.
Receive the latest infographics, guides, and blog updates for medical device manufacturing, package testing, and sterilization.