Validated cleaning, decontamination, and inspection services for reusable and single-use medical devices in FDA-registered, ISO-certified facilities.

Ensuring medical devices and surgical instruments are properly cleaned, decontaminated, and inspected is critical for patient safety, regulatory compliance, and device longevity. At Life Science Outsourcing (LSO), we provide end-to-end cleaning, decontamination, and reprocessing solutions for both single-use and reusable medical devices.
As an FDA-registered, ISO 13485-certified contract manufacturer, our controlled cleaning processes, validated decontamination methods, and rigorous quality inspections help manufacturers meet regulatory standards and ensure product integrity.
Reusable Device Processing
LSO specializes in precision cleaning and reprocessing of reusable medical devices to ensure sterility, functionality, and compliance. Our capabilities include:


Reprocessing single-use devices can reduce waste and lower costs while ensuring compliance with FDA reprocessing guidelines. Our services include:
LSO provides comprehensive processing and sterilization services for medical loaner kits, ensuring fast turnaround times and full compliance. We offer:
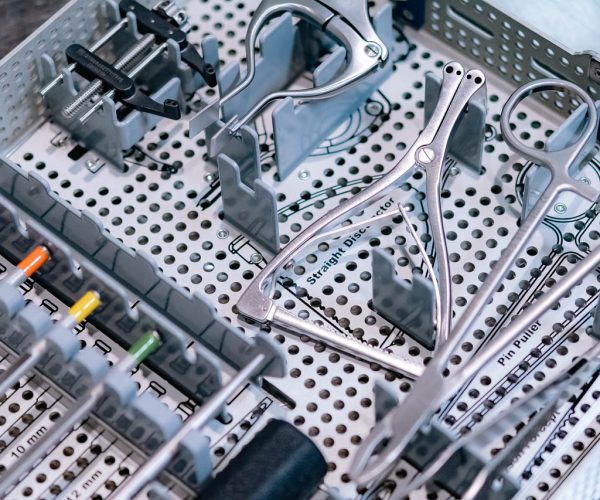

Returned medical devices and instruments must be thoroughly decontaminated, tested, and reprocessed before being reintroduced into the supply chain. LSO offers:
Every cleaned and reprocessed device undergoes rigorous quality inspection to verify sterility, functionality, and compliance. Our services include:

Life Science Outsourcing is an FDA registered and ISO 13485 certified full service Medical Device Contract Manufacturer.
We improve our products and advertising by using Microsoft Clarity to see how you use our website. By using our site, you agree that we and Microsoft can collect and use this data. Our privacy notice has more details.
Receive the latest infographics, guides, and blog updates for medical device manufacturing, package testing, and sterilization.