Speed Time-To-Market: Open Capacity, Process Efficiency, and Expert In-House Guidance

A growing number of medical device innovations are introduced each year to equip medical professionals with solutions designed to enhance patient health. A critical aspect of these devices is sterilization, which ensures the eradication of pathogens to prevent healthcare-associated infections (HAIs).
Unfortunately, many companies experience obstacles in the sterilization process due to limited capacity and outages, complex processes involving too many vendors, and difficulty adhering to standards. LSO resolves these issues with open capacity, streamlined one-stop shop, in-house microbiologist, and bio-coastal redundancy.
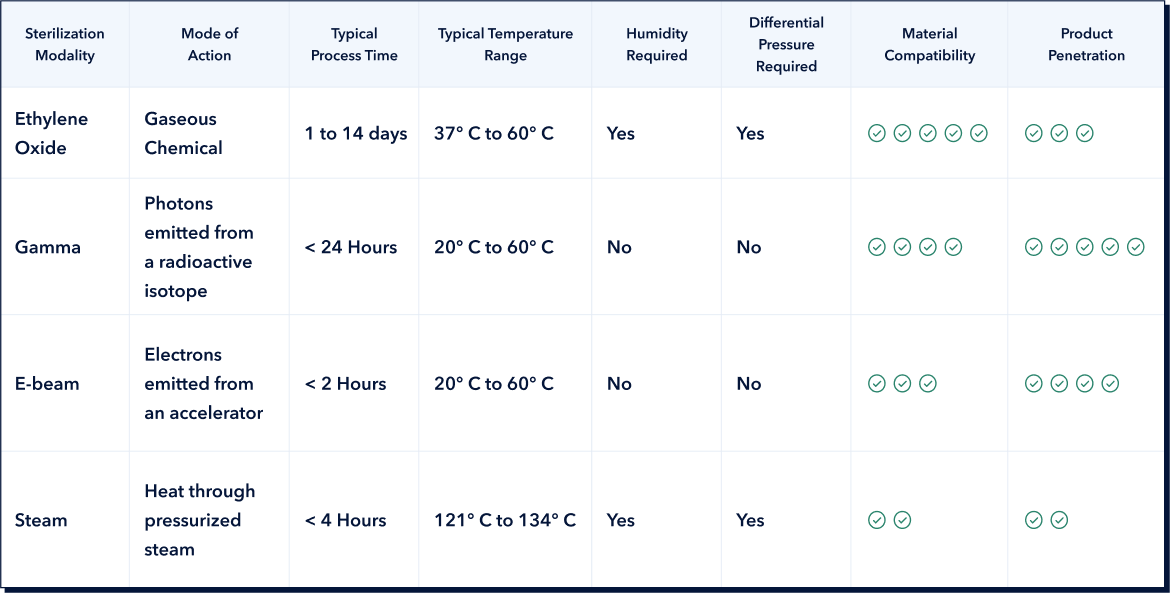
Ethylene Oxide (EtO) sterilization leverages a colorless gas that functions at relatively low temperatures. This approach currently stands out as the predominant sterilization technique for medical devices. Products are enclosed within a secure chamber where they are exposed to EtO gas. EtO inactivates microorganisms through alkylation of their molecular structures and genetic material.


This sterilization modality uses ionizing radiation in the form of high-energy photons, inflicting damage to the DNA of microorganisms and inhibiting their reproductive capabilities. Typically, gamma radiation at the irradiator emanates from the radioisotope cobalt-60 . The exposure duration within this irradiator is meticulously timed based on many variables, including the nature of the product undergoing sterilization.
Commonly denoted as ‘e-beam’ within the sterilization sector, this technique relies on high-energy electrons from an accelerator to quickly destroy microorganisms. Products are usually aligned on a conveyor system, which transports them smoothly through two opposing electron beam paths. The administered e-beam dose can be precision-calibrated to neutralize microorganisms while minimizing risks of harm to the devices themselves.


Steam sterilization is achieved through use of pressurized steam at elevated temperatures inside an autoclave. It is adept at neutralizing detrimental bacteria, viruses, fungi, and various spores that are present on devices and in pharmaceutical products. Though the use of pressurized steam is centuries-old, the modern autoclave is still widely used in industrial sterilization applications. The steam sterilization process is quick, with typical exposure times of less than one hour.
Life Science Outsourcing provides production batch processing in association with our partner, Sterilization Services, and in conjunction with our internal medical device manufacturing division. All processing is performed in accordance with ISO 11137.

Comprehensive packages for regulatory compliance.

From fractional to full cycles with EtO residual testing.

Secure with 2X qualification.

Tailored to your sterilization needs.
Receive the latest infographics, guides, and blog updates for medical device manufacturing, package testing, and sterilization.