Introduction: Why Packaging Integrity Matters
Medical device packaging integrity is critical to ensuring patient safety, regulatory compliance, and product performance. Even minor leaks in a sterile barrier system (SBS) can compromise sterility, leading to costly rework, failed validations, or product recalls.
Gross leak, or bubble leak, testing is a core component of a robust package validation strategy. Combined with other methods like dye penetration and seal strength testing, it provides assurance that packages maintain sterility throughout manufacturing, storage, and distribution.
Understanding the Sterile Barrier System (SBS)
The sterile barrier system protects medical devices from contamination. For pouches and trays, the SBS is typically formed through heat sealing or other qualified closure methods. The quality and consistency of these seals are critical to overall package integrity.
Standards such as ISO 11607-1/2 and FDA packaging guidance (21 CFR 820) outline requirements for sterile packaging systems. Gross leak testing fits into a larger validation strategy, often alongside visual inspection and dye penetration testing, providing a multi-layered approach to verify seal and package performance.
Common Causes of Package Leaks
Package leaks can result from a variety of sources:
- Seal defects: Gaps or inconsistencies, often detected through visual inspection, dye penetration, or bubble leak testing.
- Material punctures: Tiny tears or holes introduced during handling, assembly, sterilization, or shipping.
- Mishandling during assembly or sterilization: Excessive force or incorrect placement of components.
- Transit damage: Compression, impact, shock or vibration during shipping.
- Sharp objects: Components or overall shape of the device may contain areas that can puncture the package.
At LSO, we have observed instances where bubble leak testing revealed defects that were not detected by other qualitative methods such as visual inspections and dye penetration testing, reinforcing its importance as part of a multi-test validation approach.
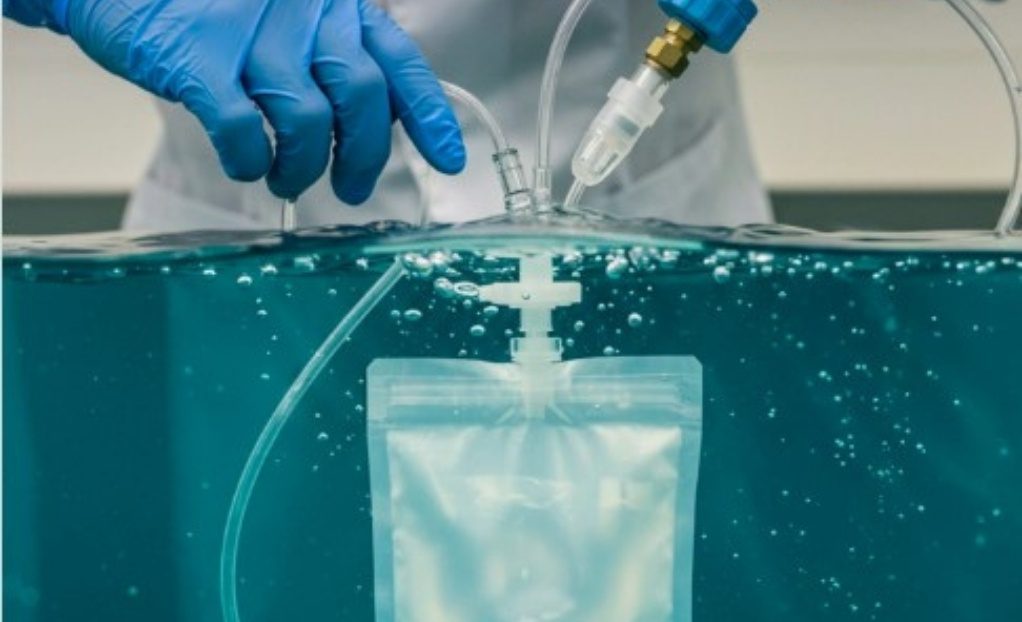
Gross Leak (Bubble) Testing Explained
Bubble leak testing is a qualitative method used to detect gross leaks in sealed packages. According to ASTM F2096, the test involves immersing a package in a liquid and applying internal pressure and controlled by detecting defects of 250 microns or greater. A consistent stream of escaping air produces bubbles indicates a leak.
Key points:
- Sensitivity: Typically detects leaks large enough to compromise sterility.
- Applications: Pouches, trays, and other package types where visual inspection or dye penetration may be insufficient.
- When to use: Both during validation and routine quality assurance.
Comparing Integrity Test Methods
| Method | Leak Location | Sensitivity | Typical Use | Regulatory Acceptance |
| Bubble Leak | Yes | Gross leaks | Pouches/trays | ASTM F2096 |
| Dye Penetration | Yes | Moderate | Seal verification | ASTM F1929, ASTM F3039 |
| Vacuum Decay | No | Fine leaks | Flexible packaging | ASTM F2338 |
| Helium Leak | No | Fine leaks | Foil pouches | ASTM F2391 |
Bubble leak testing is often compared to dye penetration because both qualify the seal. Bubble testing provides additional assurance by detecting defects anywhere in the package, not just at the seal.
Integrating Testing with Assembly, Packaging, and Sterilization
Package integrity depends on upstream and downstream processes. LSO’s end-to-end capabilities—assembly, packaging, and sterilization—enable faster troubleshooting and more efficient validation. By maintaining strict process alignment, we reduce the risk of defects, ensure compliance, and help our clients bring devices to market without delay.
Quantifying the Impact: Risks of Undetected Leaks
Undetected leaks can lead to:
- Failed validations requiring rework
- Product recalls
- Regulatory compliance issues
While actual costs vary, proactive testing minimizes the likelihood of these events, supports smooth regulatory submissions, and reduces the risk of delays or additional costs during production.
Best-Practice Checklist: Package Integrity Readiness
LSO provides a practical checklist to help engineers evaluate package readiness:
- Verify seal design and uniformity
- Conduct visual inspection of all packages
- Perform concurrent testing (dye penetration, bubble leak)
- Maintain complete validation documentation through revalidations over time
- Review test selection and method applicability
How LSO Supports Package Integrity from Start to Finish
LSO combines technical expertise, rapid turnaround, and regulatory alignment to support packaging integrity throughout the product lifecycle. While ISO 17025 accreditation is in progress (expected Q2 2026), our team already applies rigorous quality standards to all testing and validation activities.
Resources & References
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.