Interpreting ASTM D4169 Schedule I: What Low-Pressure (Altitude) Data Really Tells You
ASTM D4169 Schedule I evaluates how a packaged product responds to reduced atmospheric pressure, such as that encountered during air transport or high-mountain terrain. While often referred to as an “altitude test,” Schedule I is frequently misunderstood or over-interpreted. Its value lies not in simulating every aspect of an air or mountain shipment, but in isolating how pressure differentials affect package integrity and containment.
For medical device manufacturers, Schedule I provides insight into whether a packaging system can maintain performance when exposed to internal pressure changes that may stress seals, closures, and material interfaces.
What ASTM D4169 Schedule I Is Designed to Simulate
Schedule I represents low-pressure environments including:
- Reduced cabin pressure in cargo holds
- Rapid pressure changes during ascent and descent
- Differential pressure between the inside and outside of a package
The schedule applies a controlled vacuum exposure to simulate altitude effects. It does not simulate temperature changes, vibration, or handling in low-pressure environments—those hazards are addressed elsewhere in the distribution cycle.
Schedule I is intentionally narrow in scope. Its purpose is to evaluate pressure-driven stress, not overall shipping robustness.
What Data Schedule I Actually Produces
Schedule I produces data related to a package’s ability to withstand internal pressure expansion without loss of integrity.
Typical observations include:
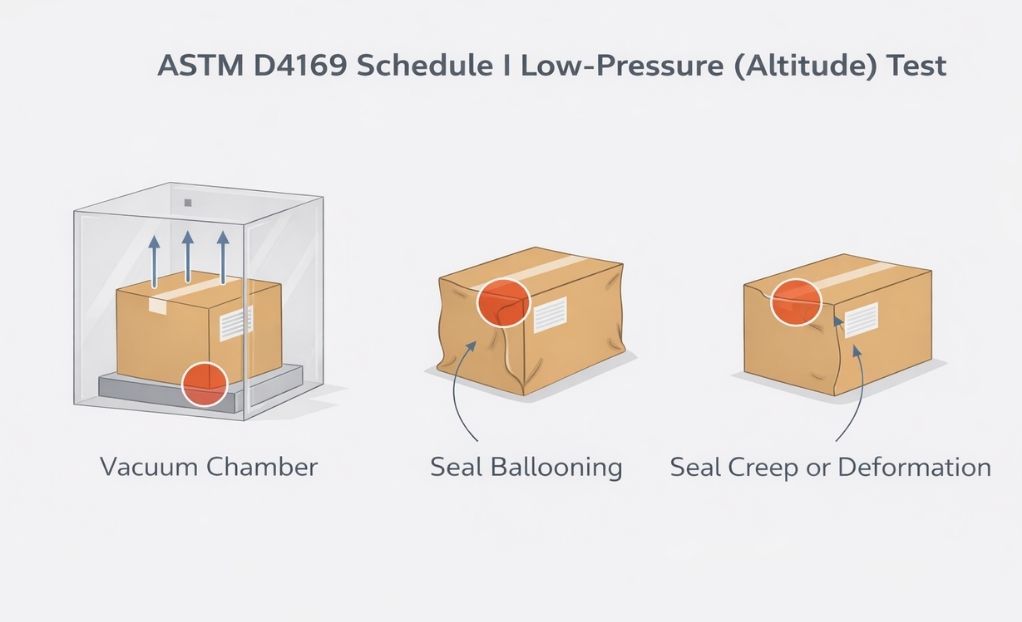
- Seal creep or elongation
- Loss of seal continuity
- Leakage of liquids, powders, or gases
- Delamination or material separation
- Changes in package geometry
Unlike drop or vibration testing, Schedule I often produces subtle but critical effects, particularly at seal interfaces.
How to Interpret Schedule I Results Correctly
- Focus on Seal Behavior, Not Just Gross Failure
Schedule I is primarily a seal stress test. Engineers should evaluate:
- Whether seals creep under pressure
- Whether deformation is elastic or permanent
- Whether seal geometry changes after pressure normalization
A seal that does not rupture but permanently stretches may still represent elevated risk.
- Pressure Effects Can Be Configuration-Dependent
Results can vary significantly depending on:
- Headspace volume
- Package orientation
- Internal contents (liquid vs. solid)
- Material permeability
Engineers should avoid assuming results are universally transferable across configurations.
- Interpret Schedule I in Sequence Context
Schedule I often appears alongside vibration or handling events. Engineers should consider whether pressure exposure:
- Weakens seals prior to vibration
- Increases susceptibility to leakage after drops
- Alters material stiffness or tension
Pressure-induced damage may not be immediately visible but can amplify downstream failures.
Interpreting “Pass” Results Without Overconfidence
A common misinterpretation is assuming that absence of leakage equates to low risk.
Instead, engineers should ask:
- Did seals deform or stretch?
- Did materials whiten or delaminate?
- Did the package feel more compliant after testing?
These subtle indicators may signal reduced design margin even if acceptance criteria are met.
Schedule I as a Diagnostic Tool
Schedule I is particularly useful for evaluating:
- Seal strength margins
- Headspace assumptions
- Material selection for air shipment
- Closure and containment design
It is often applied selectively, especially when air shipment or high terrain is expected or unavoidable.
Common Misinterpretations of Schedule I
Schedule I is frequently misused when teams:
- Treat it as a substitute for burst testing for non-porous packaging
- Assume it applies to all package types equally
- Ignore deformation that does not result in leakage
- Apply unrealistic pressure levels without justification
Schedule I is a pressure-specific diagnostic exposure.
How Auditors and Regulators View Schedule I Data
Auditors and regulators typically view Schedule I as:
- Evidence of pressure tolerance
- Supporting data for air shipment or high terrain risk assessment
- A targeted evaluation of seal and containment integrity
They expect:
- Clear justification for pressure conditions
- Documentation of deformation, not just leakage
- Logical interpretation tied to risk
Schedule I data becomes problematic only when it is used to justify claims beyond pressure performance.
Schedule I and Packaging Assurance Level
In medical device packaging validation, assurance level reflects confidence that a packaging system will maintain its intended performance throughout distribution based on the combined evaluation of multiple hazards.
Schedule I alone does not establish an overall assurance level. Its value lies in strengthening confidence when interpreted alongside handling, compression, and vibration data in alignment with ISO 11607 and risk management principles.
When Schedule I findings are well-documented and integrated with other test results, they reduce uncertainty and improve the defensibility of validation decisions involving air shipment.
Expert Perspective from LSO
“Schedule I is where pressure exposes seal assumptions. Even without leaks, seal stretch or deformation can tell you whether your package really has margin for high altitudes.”
— Matthew Emrick, Packaging Specialist, Life Science Outsourcing
The Real Value of Schedule I
ASTM D4169 Schedule I is not intended to replicate every aspect of a low pressure environment. Its strength lies in isolating pressure-driven stresses that directly affect seal and containment performance.
When interpreted correctly, Schedule I:
- Identifies high altitude-related vulnerabilities
- Improves seal and headspace design
- Supports risk-based validation decisions
- Strengthens overall distribution assurance
Final Engineering Perspective
Schedule I should be viewed as a pressure-stress lens within ASTM D4169. It does not replace handling, vibration, or compression testing, nor does it independently define distribution robustness.
Its true value emerges when pressure effects are interpreted alongside other hazards to build a defensible, risk-based packaging validation strategy.