Medical device, pharmaceutical, and biotech companies must follow stringent medical device packaging validation processes to obtain what they need for an FDA or EU submission: The coveted validation report.
Your terminally sterilized medical devices cannot be launched to market without proper packaging and a validation report proving they will remain sterilized until opened for initial use. You might be ready for that stage of your device project and have questions. What’s involved with validation to get the validation report? What does ISO need from a packaging validation standpoint? Such questions are best answered by looking closely at ISO 11607.
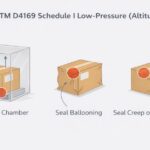
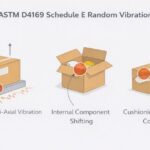
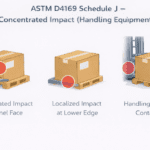
The International Organization of Standardization (ISO) established 11607 for device manufacturers to demonstrate the potency of their sterile barrier packaging. The sterile barrier packaging must prove resilient enough to withstand various tests: Environmental, distribution, and accelerated aging. A medical package testing company can perform this testing in a controlled environment and also perform package strength and integrity testing. Package strength and integrity testing ensures the package will not only survive its testing challenges but continue to maintain a sterile barrier. That is what will meet ISO 11607 criteria.
ISO 11607 impacts medical device packaging in two primary ways. Those are 11607-1 and 11607-2.
What is ISO 11607-1?
ISO 11607-1 outlines requirements for materials, sterile barrier systems, and packaging systems of devices that must maintain sterility until point of use. Requirements and tests cover:
- Materials
- Preformed sterile barrier systems
- Sterile barrier systems
- Packaging systems
What is ISO 11607-2?
ISO 11607-2 outlines validation requirements for forming, sealing, and assembly processes. They are crucial to ensure that sterile barrier system integrity can be maintained until opened by the users of sterile medical devices.
What do medical device companies need to know for package testing?
Medical device companies know they need the validation report. To create that report, they need to work with a medical package testing company to test according to ISO 11607. That company should help you with the goals of a terminally sterile medical package system:
- Package system performance testing / Distribution simulation
- Stability testing / Shelf-life study
- Package system integrity testing
You should also be aware that testing can include:
- Visual inspection test
- Peel strength test
- Burst test
- Dye penetration test
- Creep test
- Bubble emission test
Writing a testing protocol
Medical device companies often ask about the testing protocol. Those protocols are required for FDA, MDD, or other type of regulatory submission. Will the medical package testing company resource write it? The answer may be yes. At Life Science Outsourcing, Inc. we’ve spent years writing protocols for large and small companies.
The testing protocol should describe:
- What will be tested
- What type of testing will be performed
- Acceptance criteria for each test
- Standards each test will be run to
Identify the right medical device testing partner
Medical package testing can seem to be a daunting process. But it doesn’t have to be. The right medical package testing partner should be able to navigate ISO 11607 with you, answer your questions, provide guidance on protocol, and your final report.
Learn more about the Life Science Outsourcing Medical Package Testing (MPT) division.