There is no question that medical device manufacturers have experienced unprecedented supply chain challenges in recent years. Pandemic-related shortages, distribution roadblocks and more are responsible for setting many companies back – and the struggle remains today as new difficulties continue to arise.
However, there is a secret that many supply chain leaders have uncovered and use effectively today to their advantage, giving them the ability to stay agile and yet consistent with every aspect of their job. They have discovered that working with an integrated service provider partner in the manufacturing space can definitively meet their needs across an expansive ecosystem of services.
Contract manufacturing organization strengths
A contract manufacturing organization with integrated services can ensure that supply chains achieve the top three necessary requirements for reliable continuity. These include resiliency, consistency, and shorter time to market.
Supply chain resiliency
2024 will continue to present new challenges to supply chain processes. New requirements, resource access limitations and more can be expected. Supply chain leaders can face those challenges one at a time and with considerable time investment – or they can align themselves with a dedicated contract manufacturing organization capable of successfully owning many of those responsibilities. Such a partner with an advanced ecosystem of services across packaging, sterilization, and distribution can navigate industry changes, complexities, and requirement updates with ease. That in turn creates resiliency for device companies and their supply chain processes to weather ever-evolving issues.
Supply chain consistency
Getting product out the door to its destination can be impacted by dozens of different types of processes. In many cases, utilizing several resources to achieve supply chain continuity can become a roadblock in and of itself. It means companies must juggle multiple timelines, disparate materials or resources access, and detrimental limitations of in-house expertise. Working with one contract manufacturing organization capable of managing the medical device process with end-to-end services allows companies and their supply chain to anticipate and resolve issues quickly, to pivot as needed to prevent negative outcomes, and ultimately achieve consistent results over time.
Time to market
Supply chain professionals are under constant scrutiny to hit target goals. Product must get to market quickly. Yet the myriad of daily logistical, regulatory, and industry challenges create persistent obstructions that contribute to delays. Managing all of them through different channels is a daunting task with the inevitable outcome of increased risk to the bottom line or even the end user. But it doesn’t have to be this way. Manufacturers can rest easy when working with one contract manufacturing organization offering an expansive ecosystem of services – keeping a project under one roof ensures it moves through each service quickly with more effective communication between departments and services to be distribution-ready on time.
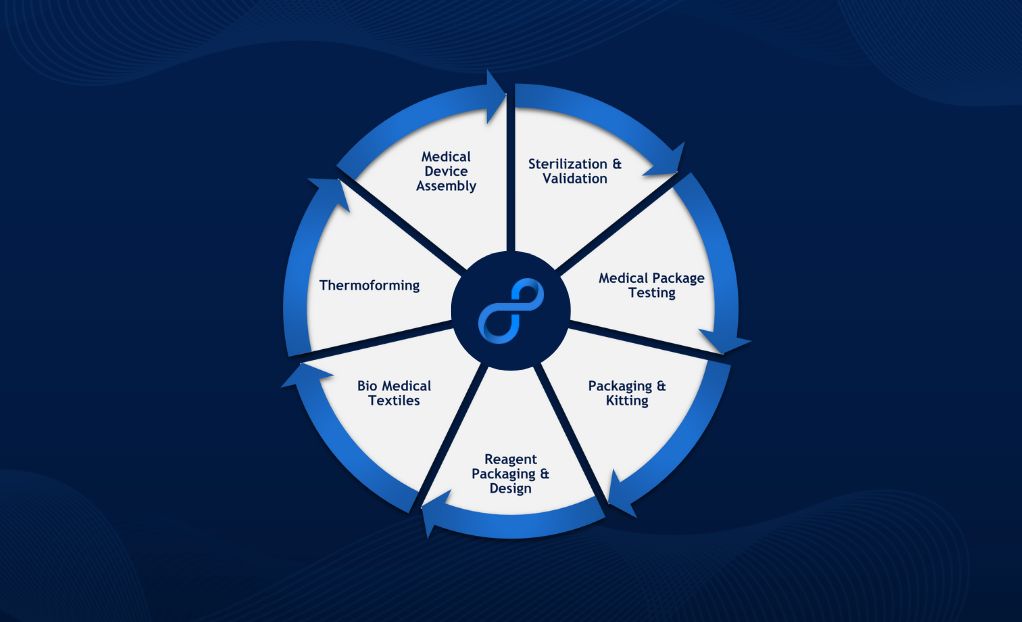
Life Science Outsourcing helps bring medical innovations to life with all the service expertise needed for companies to achieve supply chain resiliency, consistency, and shorter time to market:
- Medical device assembly
- Sterilization and validation
- Medical package testing
- Packaging and kitting
- Reagent packaging and design
- Biomedical textiles
- Thermoforming
Are you ready to achieve a reliable supply chain continuity? Learn more about how working with a contract manufacturing organization like LSO can resolve supply chain challenges and set companies up for a more successful 2024 – and beyond. Contact us today to get started.
Looking for more information on this topic? Read 2024 Medical Device Supply Chain Strategies Require These Pivotal Approaches to Succeed.
Start up. Speed up. Scale up. Founded in 1997, Life Sciences Outsourcing is an FDA-registered and ISO 13485-certified organization with services and capabilities spanning the entire medical device product life-cycle – from turnkey manufacturing, testing, validation, and sterilization to precision packaging, fulfillment, and distribution. Email us at info@lso-inc.com or call (714) 672-1090 today to get started.