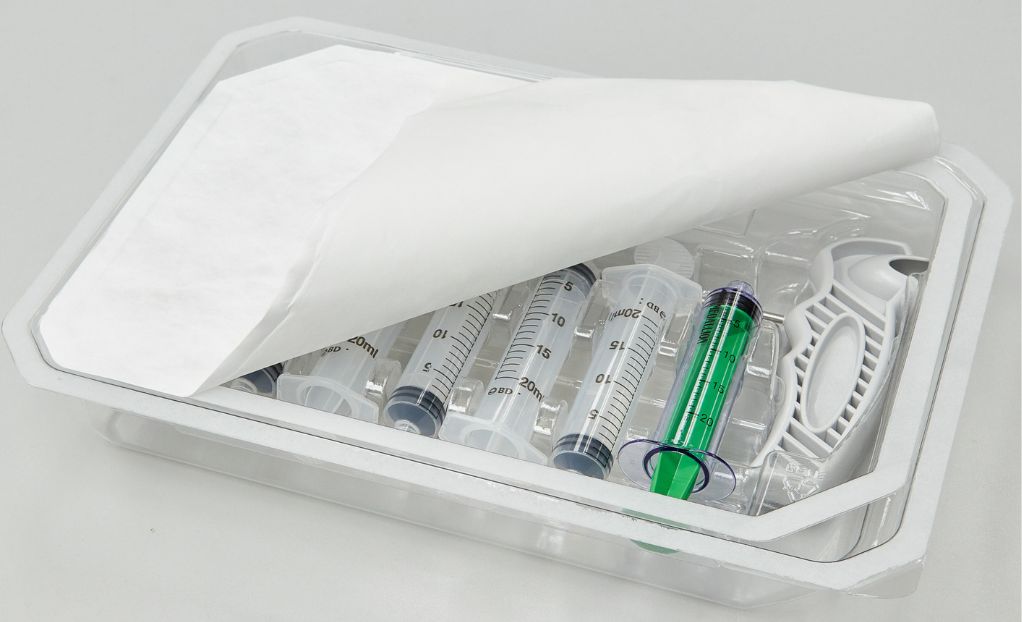
Medical device manufacturing has extensive and rigorous requirements to ensure patient safety and prevent infections. Device sterility is a key part of the process, as is the packaging to maintain that sterility – the ultimate patient safeguard. The packaging sterile barrier plays a critical role in maintaining a device’s sterility until it is ready to be used. Let’s explore what the sterile barrier is, how it is validated, and why validating its effectiveness is an essential step in the manufacturing process.
What is a medical device packaging sterile barrier?
A sterile barrier is the protective enclosure surrounding a medical device that prevents any contamination from occurring throughout its shelf life or the distribution process and lasting until the package is opened for use. It is an industry standard as outlined in ISO 11607-1: 2019 Requirements for Materials, Sterile Barrier Systems and Packaging Systems. There are typically multiple layers of packing materials, including primary, secondary, and tertiary packaging. Primary packaging is in direct contact with the device and acts as a physical barrier against pathogens. Secondary packaging provides additional protection, and tertiary packaging is used for bulk shipping and storage.
The importance of validating the packaging sterile barrier
Validating (or confirming) the packaging sterile barrier is achieved by proving its integrity over the medical device’s shelf life and throughout the distribution lifecycle via specific validation testing methods. These can include:
- Visual inspection test
- Peel strength test
- Burst test
- Dye penetration test
- Creep test
- Bubble emission test
Reasons for validating the packaging sterile barrier, beyond meeting existing regulatory requirements, are a compelling list:
Patient safety
By validating the packaging, manufacturers can confirm that the device remains sterile until it reaches the end user, which is necessary to prevent infections and reduce potential patient harm during medical procedures.
Regulatory compliance
Regulatory authorities require medical device manufacturers to validate sterile barrier packaging. Compliance with these regulations is essential for obtaining regulatory approvals and maintaining compliance while demonstrating the manufacturer’s commitment to stringent quality standards.
Quality assurance
Validating the sterile barrier is an integral part of quality assurance in medical device manufacturing. The process allows manufacturers to verify that the packaging materials, design, and processes effectively maintain device sterility throughout shelf life and distribution. It allows the packaging to meet industry standards and best practices, reduces the risk of contamination, and provides consistent product quality.
Risk mitigation
Validating the sterile barrier helps identify potential risks and vulnerabilities in the packaging system. By conducting rigorous testing and validation processes, manufacturers can detect any weaknesses in the packaging and make necessary improvements. This minimizes the risk of package breaches or compromised sterility, protecting both the device and the patient.
Product integrity
The sterile barrier not only prevents microbial contamination but also safeguards the medical device from physical damage, moisture, and other environmental factors. Validating its effectiveness verifies that the packaging adequately protects the device and maintains its physical and functional integrity until it reaches the end user.
Confidence and trust
Validating the sterile barrier and adhering to regulatory requirements instills confidence in healthcare professionals and their patients. It assures them that the medical device has been properly packaged and that stringent quality control measures have been completed. The process demonstrates a manufacturer’s commitment to patient safety and product reliability.
Preventing infections and maintaining end user safety is paramount in medical device manufacturing, which is why ensuring medical device sterile barriers and their integrity to withstand storage and distribution timelines and activities is so important. While the packaging can offer an effective design that seems to support maintaining the sterile barrier, it must also be validated to mitigate risks and ensure product integrity. This fosters confidence and trust in your end users and demonstrates a commitment to upholding the highest quality standards and prioritizing patient well-being.
Looking for more information on microfluidic medical device packaging? Read Preventing Damage and Maintaining a Sterile Barrier: Why Medical Device Testing is Necessary.
Start up. Speed up. Scale up. Founded in 1997, Life Sciences Outsourcing is an FDA-registered and ISO 13485-certified organization with services and capabilities spanning the entire medical device product life-cycle – from turnkey manufacturing, testing, validation, and sterilization to precision packaging, fulfillment, and distribution. Email us at info@lso-inc.com or call (714) 672-1090 today to get started.