ASTM-F1980
ASTM F1980 Accelerated Aging for Medical Device Packaging
ASTM F1980 is commonly used during medical device packaging validation to estimate product expiration dates while real-time aging studies are still in progress. Results generated under ASTM F1980 are considered conservative and must ultimately be confirmed through real-time aging in accordance with ISO 11607.
This standard applies to a wide range of packaging systems, including pouches, trays, and other sealed medical device packaging configurations. ASTM F1980 does not replace real-time aging but serves as an early validation tool to support regulatory submissions and market release timelines.
This page provides an overview of ASTM F1980 and its role in accelerated aging of medical device packaging. For recent revisions or updates to the standard, see our summary of ASTM F1980 updates.
ASTM F1980 Video Overview
What ASTM F1980 Evaluates
Accelerated aging per ASTM F1980 models the chemical and physical changes that occur in packaging materials over time. The goal is to determine whether packaging can continue to maintain:
- Sterile barrier integrity
- Seal strength stability
- Material durability
- Component performance
Q: What is the purpose of accelerated aging?
A: To predict long-term packaging performance using data generated under elevated temperatures.
The Scientific Basis: Q10 and Arrhenius Concepts
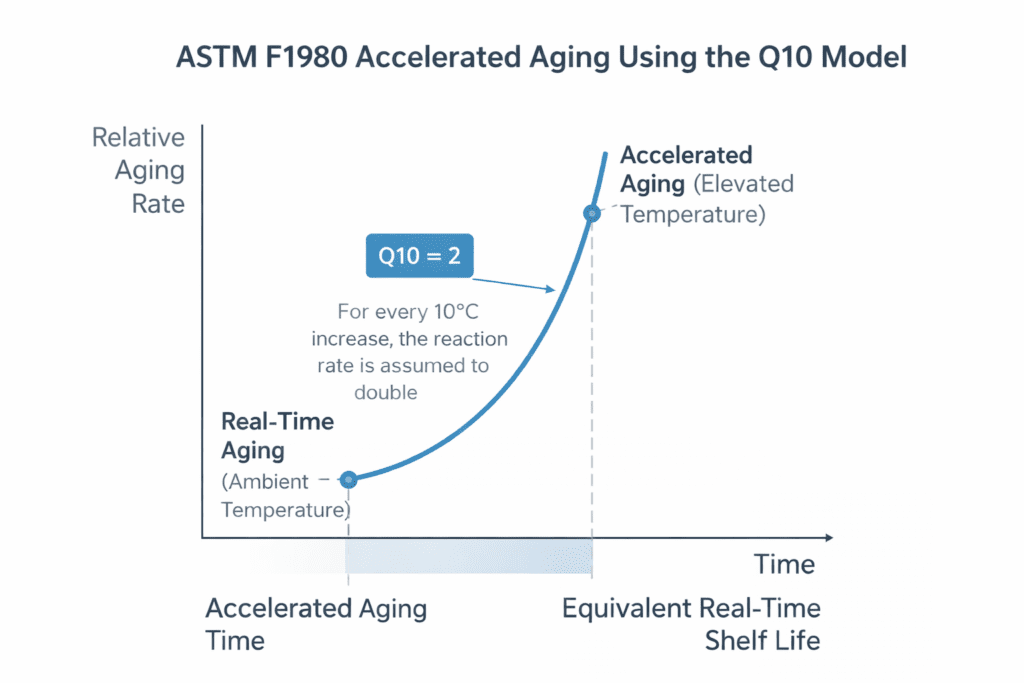
ASTM F1980 relies on the Arrhenius reaction rate theory, which describes how chemical processes accelerate at higher temperatures. The standard simplifies this through a Q10 value, the factor by which a reaction rate doubles for every 10°C rise in temperature.
Most medical device manufacturers use Q10 = 2 unless material science data supports another value.
Accelerated Aging Time Formula
AA Time = Real-Time Shelf Life / Q10^((AA Temp – Ambient Temp) / 10)
Example Calculation
Simulating 2 years of aging at an ambient temperature of 25°C with an aging temperature of 55°C:
AA Time = 730 days / 2^((55–25)/10)
AA Time = 730 / 2^3
AA Time ≈ 91 days
Q: Why is Q10 = 2 commonly used?
A: It offers a conservative, widely accepted estimate for most medical packaging polymers.
Accelerated Aging Time vs. Real Time Shelf Life
Establishing an Accelerated Aging Plan
ASTM F1980 requires manufacturers to:
- Define the claimed shelf life
- Select an appropriate Q10 value
- Choose a justified aging temperature
- Calculate the accelerated aging duration
- Age samples for the required minimum time
- Perform post-aging evaluations using appropriate test methods
Q: What happens after accelerated aging is complete?
A: Packaging systems undergo the appropriate integrity and strength testing defined by other applicable standards.
Material and Temperature Considerations
Temperature selection must account for known sensitivities of packaging materials. Excessively high temperatures may cause:
- Seal degradation
- Polymer softening or embrittlement
- Distortion of thermoformed structures
- Compromised adhesive systems
ASTM F1980 advises choosing temperatures that accelerate aging without altering material properties through mechanisms unrelated to real aging.
Q: What is the typical temperature range?
A: Most studies use 50–60°C, justified through material stability data, with the most common temperature being 55°C.
Real-Time Aging Requirements
Accelerated aging alone does not validate shelf life. ASTM F1980 states that real-time aging must occur in parallel to confirm that accelerated aging predictions accurately represent long-term material performance.
Real-time aging:
- Occurs at ambient conditions
- Matches the claimed shelf life duration
- Provides final confirmation for regulatory submissions
Q: Why is real-time aging still required?
A: Because accelerated aging provides a prediction, not empirical long-term performance data.
Regulatory Alignment
ASTM F1980 is widely accepted for shelf-life justification in:
- ISO 11607 packaging validation
- FDA 510(k) submissions
- Global regulatory pathways
ASTM F1980 FAQs
What does ASTM F1980 determine?
The predicted shelf life of sterile barrier systems using temperature-based accelerated aging.
Does accelerated aging replace real-time aging?
No. Real-time data must eventually be collected.
What Q10 value should be used?
A Q10 of 2 is typical unless materials justify a different value.
What tests follow accelerated aging?
Post-aging evaluations must follow applicable package integrity and strength test standards.
ASTM F
All Standards

Takes about 60–90 seconds.
Typical response within 1 business day.
15-minute consult with a packaging specialist.
