ASTM-F88
ASTM F88 Seal Strength Testing for Medical Device Packaging
ASTM F88 / F88M is the industry-standard test method for measuring seal strength in flexible sterile barrier systems. It confirms the mechanical integrity of a package seal after manufacturing, sterilization, transit simulation, and shelf-life aging.
Under ISO 11607, seal strength testing is a required component of sterile barrier validation.
At Life Science Outsourcing (LSO), we perform ASTM F88 seal strength testing as part of medical package validation and shelf-life programs, using calibrated tensile equipment and validated procedures.
Video Overview: ASTM F88 Explained
What ASTM F88 Measures
ASTM F88 evaluates the force required to peel open a sealed joint, providing quantitative evidence of:
- Seal uniformity
- Adequate adhesive bonding or thermal seal strength
- Package robustness after sterilization
- Long-term performance following accelerated or real-time aging
- Compliance with ISO 11607 packaging validation requirements
Q. What does ASTM F88 measure?
ASTM F88 measures the strength of the seal itself, not the material. It determines whether a sealed joint will maintain sterile integrity during the product’s expected shelf life.
Q. Is ASTM F88 required?
Yes. ASTM F88 is a recognized method for demonstrating sterile barrier performance under ISO 11607 and FDA expectations.
Why Seal Strength Matters in Packaging Validation
Seal strength is central to sterile barrier performance. A seal that is too weak may open prematurely, while one that is too strong may tear the material instead of peeling cleanly.
F88 results support:
- Sterilization validation (EtO, Steam, Gamma, eBeam)
- Accelerated and real-time aging shelf-life studies
- Transit simulation testing (ASTM D4169, ISTA)
- Design verification and process validation
Q. What is an acceptable seal strength?
There is no universal minimum value. Acceptance criteria are set by the manufacturer based on material, seal process capability, and risk management.
Q. Does ASTM F88 replace burst testing?
No. F88 measures peel strength. Burst testing (ASTM F1140/F2054) measures pressure resistance.
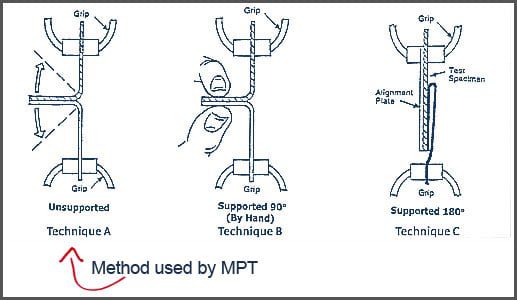
ASTM F88 Test Techniques A, B, and C
ASTM F88 provides three test configurations. LSO documents all three but performs Technique A (Unsupported), the most commonly used method for flexible sterile barrier systems.
Technique A — Unsupported
The specimen is pulled apart without external support.
- Most reflective of real-world opening
- More sensitive to operator technique
- LSO uses this method for customer studies
Technique B — Supported
The seal is supported by a plate to eliminate bending.
Technique C — Restrained
The specimen is clamped between rigid plates with an opening to expose only the seal.
These three techniques measure the same property but may produce different numerical results.
Q. Which technique should I use?
For most pouches and Tyvek systems, Technique A is preferred. Technique B or C may be used when very thin films or stiff laminates require more controlled peel geometry.
How ASTM F88 Testing Works (Step-by-Step Example)
- Condition Samples
Samples are conditioned to ambient laboratory conditions prior to testing. - Prepare Test Strips
Cut uniform 1-inch (25 mm) wide strips perpendicular to the seal area and about 3 inches long. The width of the strip can vary based on regulatory requirements or working area of the package. - Load Specimen
Insert each end of the strip into the grips of a calibrated tensile tester. - Set Crosshead Speed
LSO uses a speed of 250 mm/min (placeholder). Generally 200-300 mm/minute are used and can be customized. - Pull to Seal Separation
The instrument measures force as the seal peels open. - Record Data
Report:- Maximum force (seal strength)
- Average force (seal strength)
- Force standard deviation
- Document Failure Mode
- Adhesive Peel (which is the ideal peel)
- Elongation
- Delamination
- Material tear
- Cohesive failure
- Incomplete seal (channels, seal creep, or narrow seals)
Q. What jaw speed should I use?
ASTM F88 recommends 200–300 mm/min. LSO uses 250 mm/min unless otherwise specified.
Q. How should F88 results be reported?
Maximum, average, the standard deviation, and sample failure mode.
Sample Size & Seal Location Requirements
One of the most overlooked elements of F88 is sample planning.
For compliance with ISO 11607, you must test:
- Multiple seal locations (3 manufacturer’s seal and 1 production seal locations are most typical)
- Multiple samples per location (usually 30 or 60 depending on confidence and reliability calculations)
- Samples from each production lot
- Samples from each aging time point
Recommended minimums:
- 30–60 replicates per seal location
- Time-point testing aligned with accelerated or real-time aging
- Representative sampling for transit simulation
Q. How many samples do I need?
There is no fixed ASTM rule. Industry practice is 30–60 samples per location, per time point.
Q. Should I test across the entire seal?
Yes. It is best practice to test at least four locations to capture variability.
Common Seal Failure Modes
ASTM F88 requires documenting the type of seal failure. These modes provide insight into process capability:
- Adhesive Peel – Clean, expected opening
- Elongation – material stretches until seal peels or material breaks
- Material Tear – Film tears before seal separates
- Delamination – Laminates separate; indicates material issue
- Cohesive Failure – Seal adhesive releases prematurely
- Incomplete Seal / Channels – Process defect requiring immediate correction
Q. What failure modes are acceptable?
A clean peel is typically desired. Channels or incomplete seals, material breaks, elongation, and cohesive peels indicate failure.
Equipment Requirements
LSO uses calibrated tensile equipment designed specifically for medical package testing with:
- Precision load cells
- Pneumatic or mechanical grips
- Jaw alignment controls
- Verified speed accuracy (200-300 mm/min)
- Routine calibration and preventive maintenance

Q. Can any tensile tester perform ASTM F88?
No. The instrument must meet speed accuracy and load accuracy defined by the standard.
ASTM F88 and ISO 11607 Compliance
Seal strength is explicitly required under ISO 11607-1 as part of sterile barrier system performance testing.
ASTM F88 supports:
- Design validation
- Shelf-life validation
- Package validation
- Post-sterilization integrity verification
Regulators expect F88 data in:
- 510(k) submissions
- EU technical documentation
- Change control justifications
- Aging study reports
Q. Is F88 required for ISO 11607?
Yes — seal strength testing is mandatory.
Frequently Asked Questions (FAQs)
What does ASTM F88 measure?
Peel strength of a sealed joint.
Which technique does LSO use?
Technique A (Unsupported).
What jaw speed is recommended?
200–300 mm/min; LSO uses 250 mm/min.
Does ASTM F88 detect defects in the seal?
Yes — incomplete seals are considered failure modes.
Is ASTM F88 required under ISO 11607?
Yes — it is part of sterile barrier validation.
How many samples do I need?
Typically, 30–60 per location, per time point.
Talk to a Packaging Specialist
Get expert guidance on seal strength testing and packaging validation.
ASTM F
All Standards

