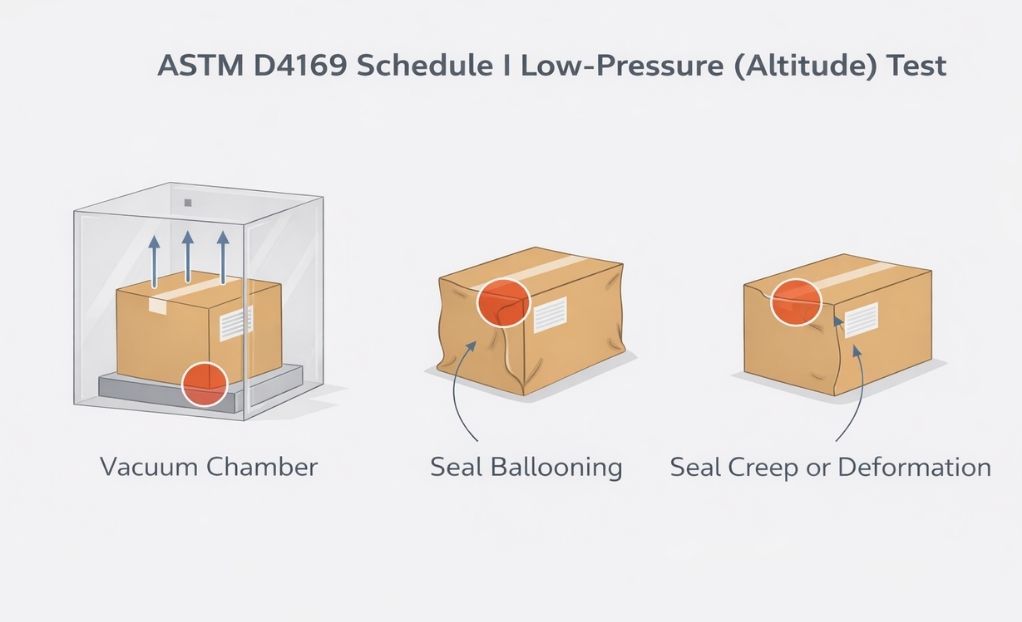
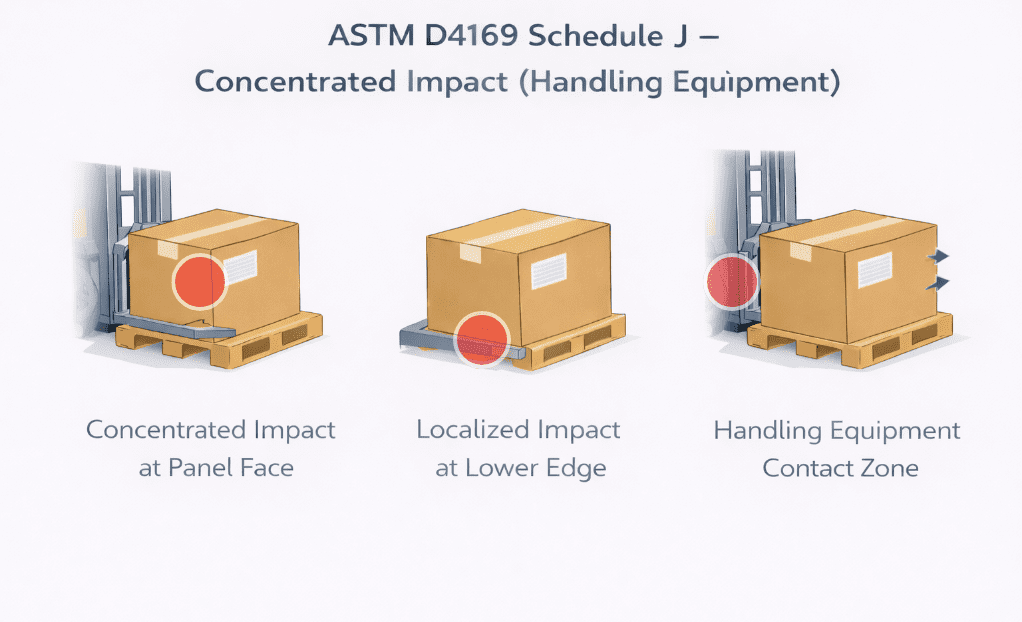
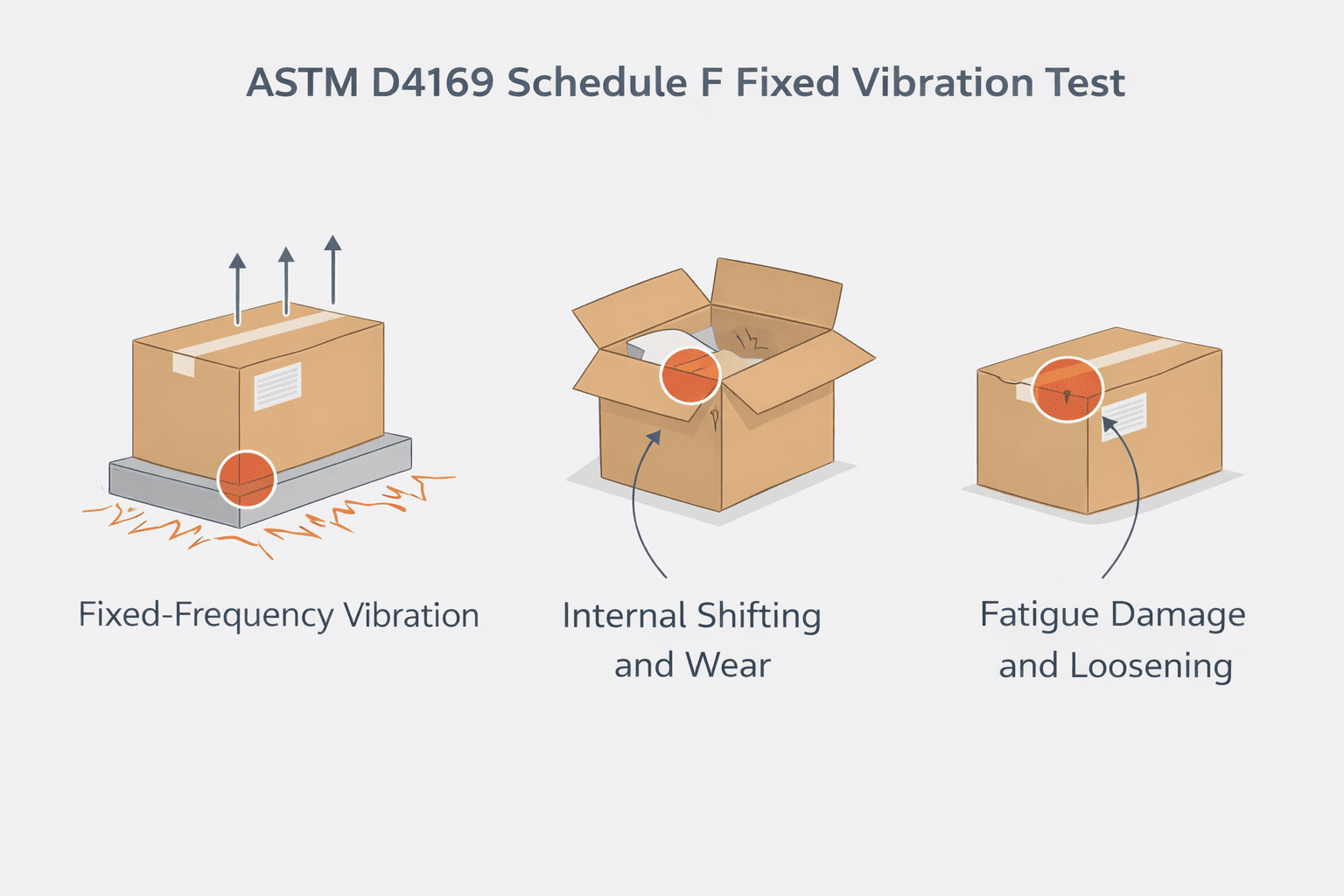
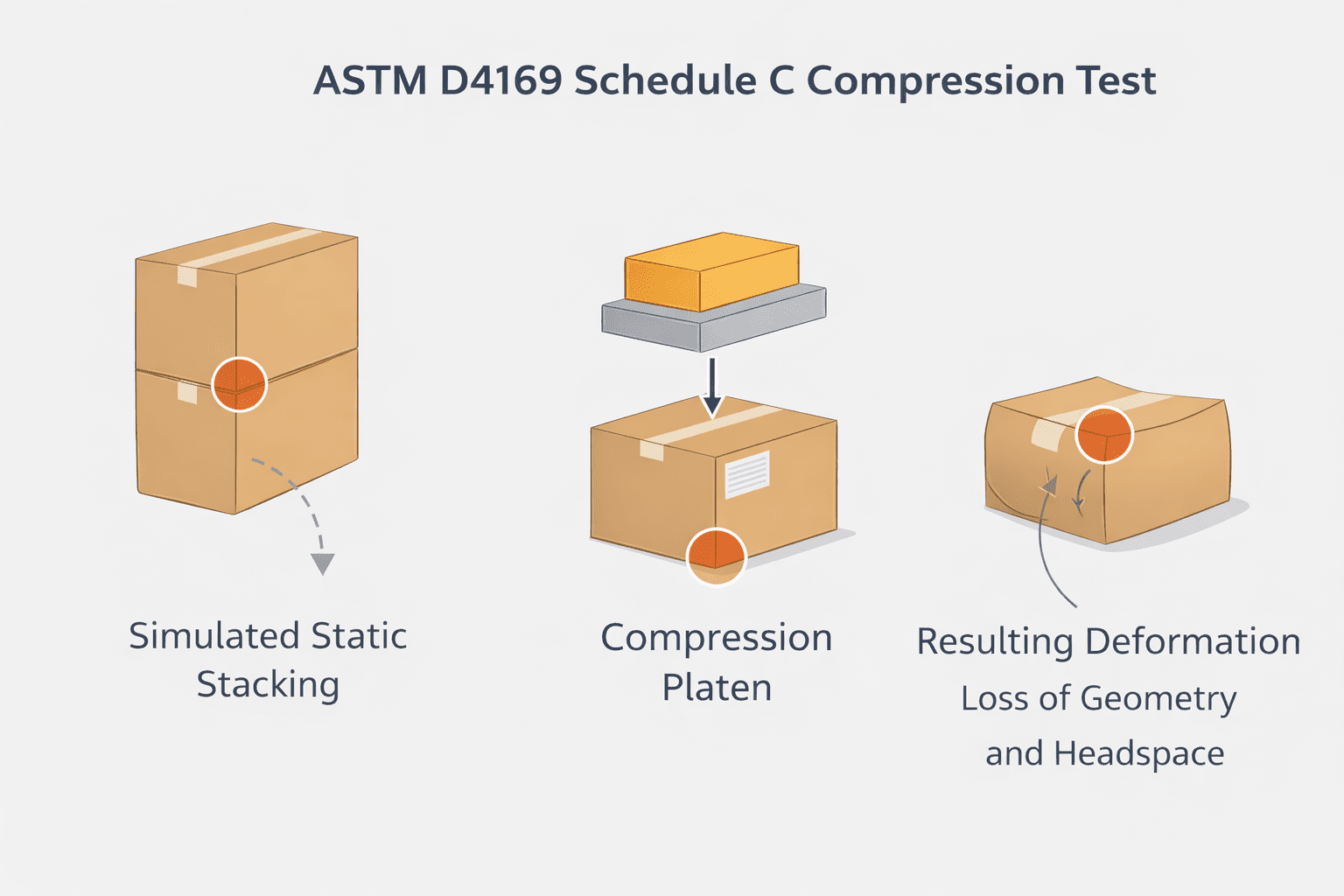
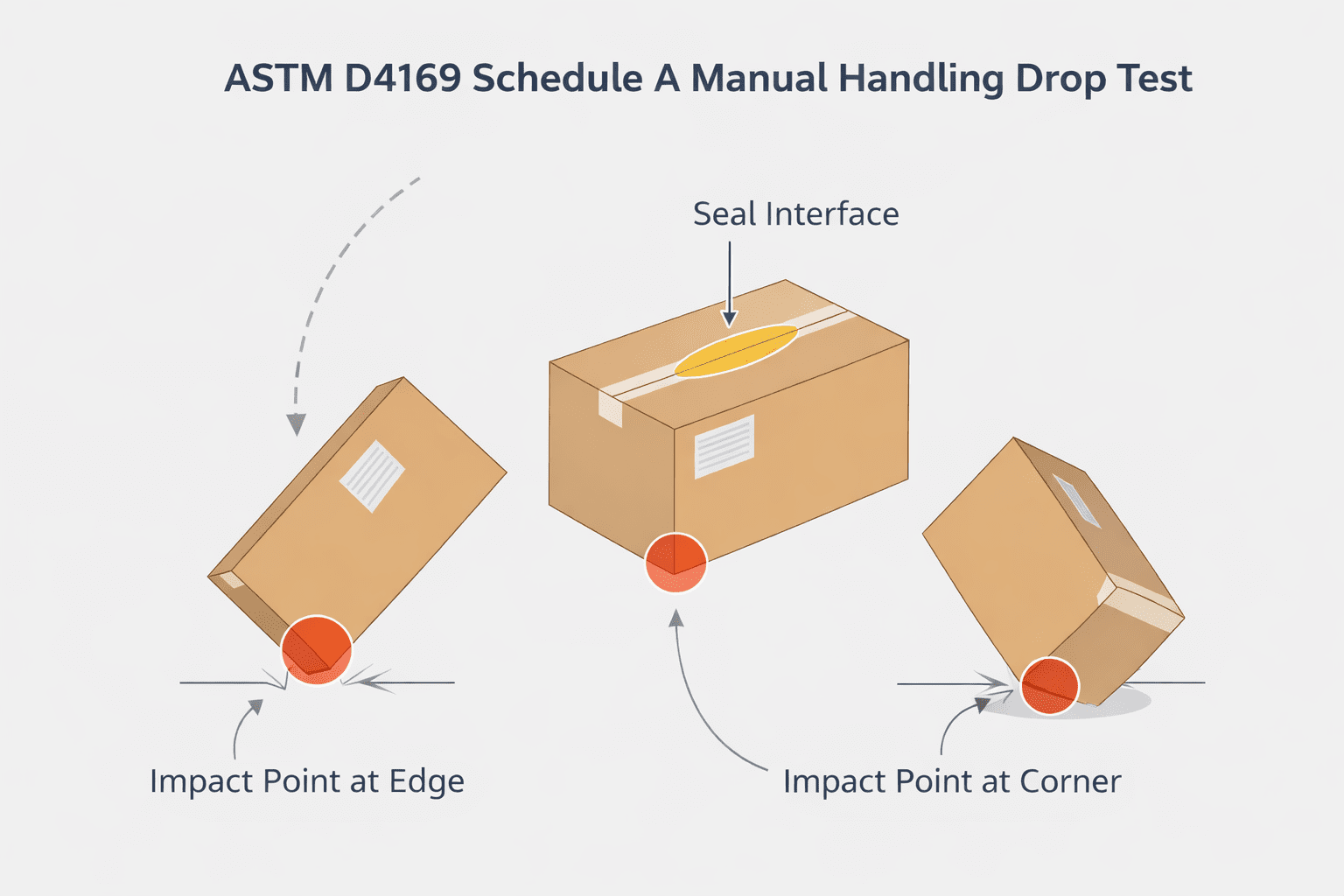
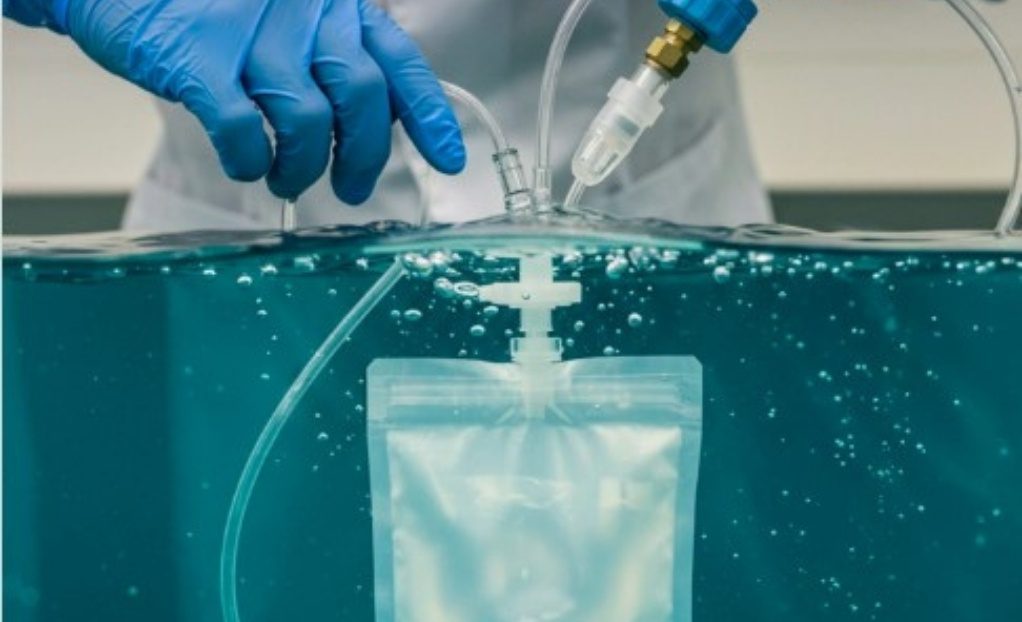
Gross leak, or bubble leak, testing is a core component of a robust package validation strategy. Combined with other methods like dye penetration and seal strength testing, it provides assurance that packages maintain sterility throughout manufacturing, storage, and distribution.