Accelerated aging is a standardized method that uses elevated temperatures to simulate the long-term shelf life of a sterile medical device package in a fraction of the time. Using the Arrhenius equation, ASTM F1980 establishes how to calculate an “accelerated aging factor” (AAF) and convert a real-time shelf-life claim—1 year, 2 years, 5 years—into a test duration measured in weeks.
To calculate aging duration correctly, manufacturers must use defensible temperature assumptions, validated Q10 values, and packaging materials proven to withstand heat. Errors in these inputs—not the math itself—are the primary reason shelf-life justifications are rejected by regulatory reviewers.
This guide explains how accelerated aging works, how to calculate aging time under ASTM F1980, and how to avoid common mistakes. It also links to LSO’s Accelerated Aging Calculator, which applies the Arrhenius equation automatically and eliminates guesswork.
Why Accelerated Aging Matters for Medical Device Manufacturers
1. ASTM F1980 Defines How Shelf-Life Simulation Must Be Conducted
Accelerated aging is used when real-time data is not yet available. The purpose is straightforward:
-
Simulate long-term storage conditions
-
Stress materials at elevated temperatures
-
Predict how the sterile barrier will perform over the claimed shelf life
ASTM F1980 is the governing standard for accelerated aging of sterile barrier systems (SBS). It establishes:
-
Acceptable temperature ranges
-
How to calculate aging duration
-
Requirements for post-aging package integrity testing
-
Documentation expectations for regulatory submissions
2. Accelerated Aging Does NOT Replace Real-Time Aging
A common misconception is that accelerated aging alone can justify a shelf life.
It can’t.
Regulatory reviewers expect:
-
Accelerated aging → initial justification
-
Real-time aging → long-term confirmation
3. Correct Calculations Are Essential for Submission Success
FDA and Notified Bodies frequently reject shelf-life claims due to:
-
Unrealistic temperature assumptions
-
Unjustified Q10 values
-
Use of temperatures that exceed material heat stability
-
Weak linkage between aging results and sterile barrier testing
Correct accelerated aging ensures that your shelf-life claim is defensible, repeatable, and aligned with regulatory expectations.
How the Accelerated Aging Calculation Works
At its core, the calculation is simple—provided your inputs are correct.
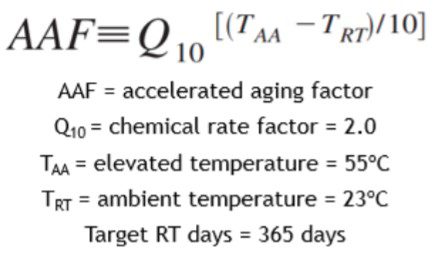
Step 1 — Use the Arrhenius-Based Aging Factor (AF) Equation
ASTM F1980 uses a simplified Arrhenius relationship:
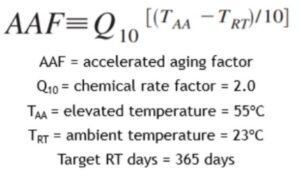
Where:
-
AAF = accelerated aging factor
-
Q10 = chemical rate factor (default 2.0)
-
TAA = elevated temperature (°C)
-
TRT = ambient temperature (°C)
The Accelerated Aging Factor tells you how many times faster materials age at an elevated temperature.
Step 2 — Convert Shelf-Life into Accelerated Aging Duration
Accelerated Aging Time=Real Time Shelf LifeAF\text{Accelerated Aging Time} = \frac{\text{Real Time Shelf Life}}{AF}Accelerated Aging Time=AFReal Time Shelf Life
Example: If 12 months of real time aging must be simulated, and AF = 9.6:
12/9.6=1.25 months≈38 days12 / 9.6 = 1.25\text{ months} \approx 38\text{ days}12/9.6=1.25 months≈38 days
Step 3 — Use a Calculator to Avoid Mistakes
Manual calculation introduces risk:
-
Incorrect decimal handling
-
Wrong Q10 coefficients
-
Confusion over Celsius vs Fahrenheit
-
Misinterpretation of TRT assumptions
LSO’s built-in calculator eliminates these issues and follows ASTM F1980 precisely.
Accelerated Aging Calculator (ASTM F1980)
Use the calculator to determine how long your accelerated aging study must run.
It applies the Arrhenius equation automatically.
Try the Accelerated Aging Calculator
The Most Common Mistakes Manufacturers Make in Accelerated Aging
Correct math doesn’t matter if your assumptions are wrong. These are the pitfalls that cause failed studies and rejected shelf-life claims.
1. Using Unrealistic Real-Time/Ambient Storage Temperatures (TRT)
Many manufacturers try to use 30°C simply because it gives a higher AAF.
But:
-
Industry standard is 22°C–25°C
-
Reviewers expect ambient conditions, not worst-case scenarios
-
Inflated TRT artificially accelerates aging and undermines credibility
If you cannot justify TRT, regulators will challenge the shelf-life claim.
2. Using an Incorrect or Unjustified Q10 Value
ASTM F1980 defaults to Q10 = 2.0, which assumes reaction rates double with every 10°C increase.
Only change Q10 if you can prove:
-
Material-specific kinetics
-
Polymer interactions
-
Packaging-specific degradation profiles
Very few manufacturers have this data.
If you’re unsure, use 2.0.
3. Choosing Temperatures That Exceed Material Heat Stability
This is the costliest mistake.
Common failure points:
-
PETG trays deform above ~60°C
-
Film/foil laminates soften unpredictably
-
Seal bonds creep, distort, or fail when overheated
“Faster aging” is not worth destroying your packaging system.
Most studies run at 55°C for exactly this reason.
4. Forgetting That ASTM F1980 Requires Sterile Barrier Testing
Accelerated aging alone is insufficient.
ASTM F1980 requires that aged samples undergo integrity testing, including:
LSO performs these tests in the same facility as aging, avoiding handoffs that create risk.
Why LSO’s Approach Produces Submission-Ready Aging Data
1. Integrated Accelerated Aging + Sterile Barrier Testing
LSO conducts:
…all under one roof.
This helps eliminate:
-
Shipping delays
-
Chain-of-custody issues
-
Testing inconsistencies
- Longer test queues
2. Continuous Monitoring and Data Integrity
Our chambers are:
-
Temperature-mapped
-
Continuously logged
-
Alarmed for excursions
-
Fully traceable
Your data isn’t just compliant—it’s defensible.
3. Study Designs Aligned with Regulatory Timelines
We help you plan:
-
Parallel AA + real-time studies
-
Submission timing
-
Packaging validation sequencing
-
Risk-based justification documents
You get a shelf-life claim that stands up to scrutiny.
Worked Examples (Show Your Math)
Example 1 — 1-Year Shelf Life at 55°C
-
TRT = 25°C
-
Q10 = 2.0
-
TAA = 55°C
AAF = 2^((55–25)/10) = 2^3 = 8
365 days ÷ 8 = 45.6 days
Example 2 — 2-Year Claim at 55°C
AAF = 8
2 years × 365 = 730 days
730 days ÷ 8 = 91.25 days
Example 3 — When Material Limits Temperature to 50°C
-
Smaller temperature delta
-
Lower AAF
-
Longer study time
Example 4 — Why 70°C Is Not Acceptable
-
Material creep
-
Seal distortion
-
Regulatory objections
-
Non-representative kinetics
This is where inexperienced ruins data.
Conclusion
Accelerated aging is only as reliable as the assumptions behind it.
Selecting validated temperatures, using an appropriate Q10, and pairing aging with sterile barrier integrity testing is the difference between a defensible shelf-life justification and a rejected submission.
LSO integrates accelerated aging, package integrity testing, and regulatory expertise under one roof—providing manufacturers with accelerated timelines, reduced risk, and submission-ready data.
If you need guidance on aging study design, or sterile barrier testing, LSO’s specialists can help.