Bubble Emission Testing
Takes about 90 seconds.
Typical response within 1 business day.
15-minute consult with a packaging specialist.
Bubble Emission (Bubble Leak) Testing for Medical Device Packaging
Bubble emission testing—commonly referred to as a bubble leak test—is a visual, destructive method used to detect gross leaks in sealed medical device packaging. The test identifies seal failures, pinholes, and material defects that can compromise sterile barrier integrity.
Bubble emission and bubble leak testing are widely used interchangeably in medical packaging, particularly when referencing ASTM F2096.
Video Overview: Bubble Emission (Bubble Leak) Testing
What Bubble Emission (Bubble Leak) Testing Evaluates
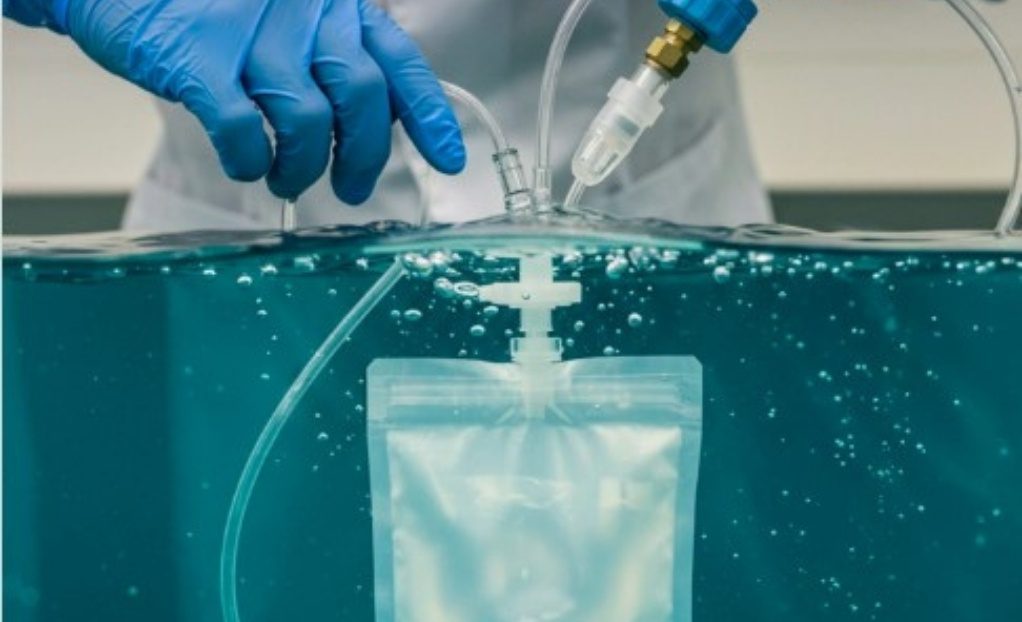
Bubble emission testing evaluates a package’s ability to maintain seal integrity by identifying:
• Gross leaks
• Seal channel defects
• Pinholes or material damage
• Package integrity failures after aging or distribution testing
Q: Is bubble emission the same as a bubble leak test?
A: Yes. Bubble emission testing and bubble leak testing are commonly used interchangeably in medical device packaging.
How Bubble Emission Testing Works
Bubble emission testing is typically performed in accordance with ASTM F2096.
General steps include:
1. Submerging the sealed package in water
2. Introducing internal pressure using air or gas
3. Observing the package for a constant stream of bubbles
4. Identifying the source and location of leakage
5. Documenting results with pass/fail acceptance criteria
If a constant stream of bubbles are observed escaping from the package, a gross leak is present.
Q: Is bubble emission testing destructive?
A: Yes. Bubble emission testing is destructive because internal pressure is applied to the package.
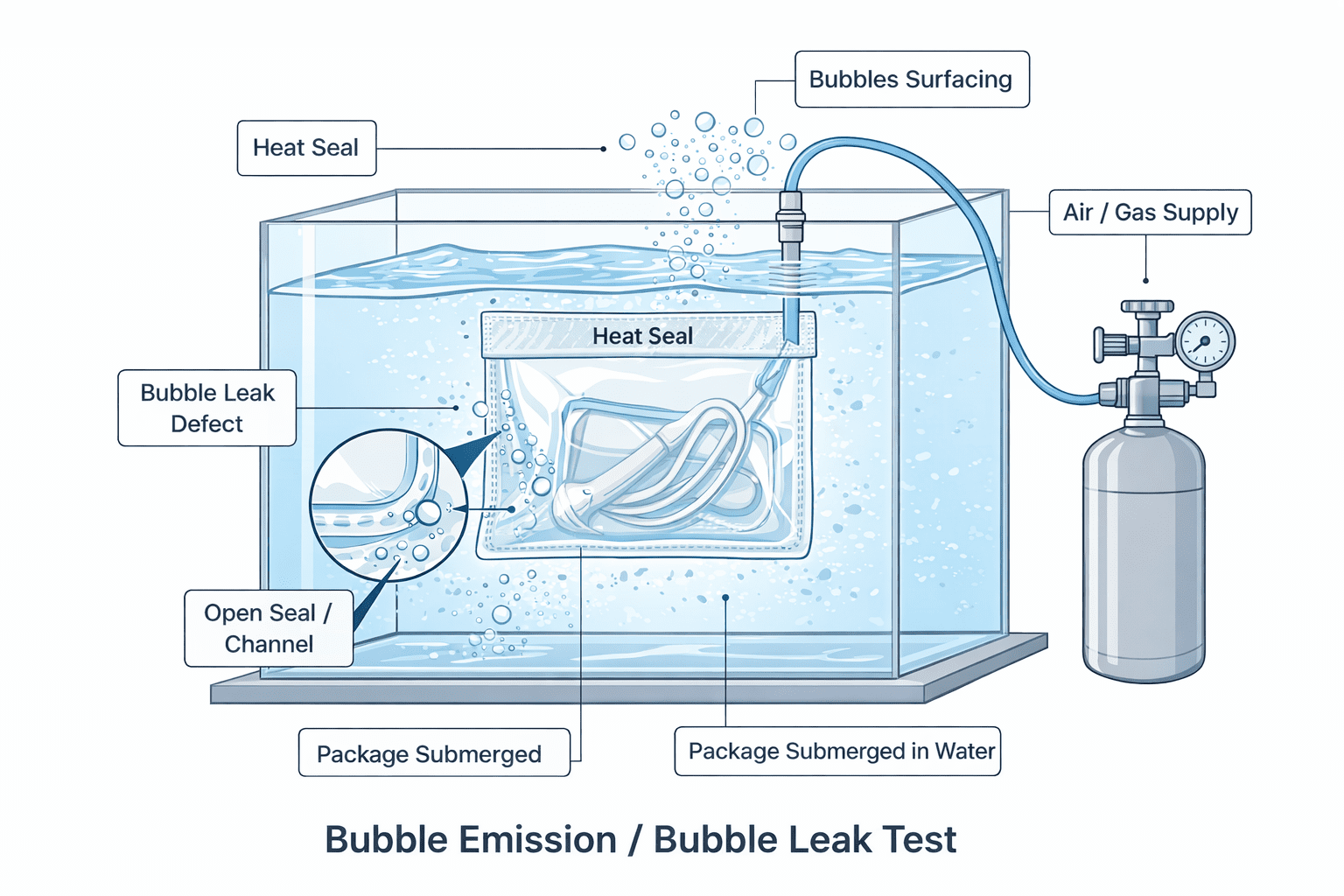
Types of Defects Detected
Bubble emission testing is designed to detect gross leaks, including:
• Open or incomplete seals
• Seal channels
• Punctures or pinholes
• Material failures
Q: Can bubble emission testing detect micro leaks?
A: No. Bubble emission testing is intended to detect gross leaks at greater or equal to 250 microns rather than micro leaks.

Packaging Types Commonly Tested
- Bubble emission testing is commonly used for:
- Flexible sterile barrier systems
- Tyvek® pouches
- Film-to-film packaging
- Paper-to-film packaging
- Medical device overwraps
- Thermoformed trays with Tyvek® lids
Q: Can rigid packaging be tested using bubble emission methods?
A: Bubble emission testing is primarily used for flexible packaging that can be pressurized; however, rigid containers with flexible lids can be tested with the proper justification.
Bubble Emission Testing Results and Interpretation
Results from bubble emission testing are typically evaluated as:
• Pass/fail outcomes
• Identification of leak location
• Confirmation of gross sterile barrier system integrity
Acceptance criteria are defined prior to testing based on validation requirements.
Q: Does bubble emission testing provide quantitative data?
A: No. The test provides a visual indication of leakage rather than quantitative leak rates. It is a pass/fail test that produces attribute data.
Role in Packaging Validation
Bubble emission (bubble leak) testing is commonly used to support:
• Packaging validation activities
• Seal integrity verification
• Distribution and aging studies
• ISO 11607 compliance strategies
Q: Does bubble emission testing support ISO 11607?
A: Yes. Bubble emission testing is widely used to support sterile barrier integrity requirements under ISO 11607.
Bubble Emission Testing FAQ
What is bubble emission testing?
A visual test used to detect gross leaks in sealed medical packaging.
Is bubble emission the same as a bubble leak test?
Yes. The terms are commonly used interchangeably.
Which standard governs bubble emission testing?
ASTM F2096 is the primary standard.
Is the test destructive?
Yes.
Takes about 90 seconds.
Typical response within 1 business day.
15-minute consult with a packaging specialist.

