Life Science Outsourcing Completes Acquisition of J-Pac Medical
Bringing Medical Innovations to Life
Life Science Outsourcing is a performance-driven contract manufacturer helping medical device and pharmaceutical innovators start up, speed up, and scale up.
Your Trusted Partner for Over 25 Years
Life Science Outsourcing has partnered with more than 1,400 medical device innovators to streamline production and navigate complex regulatory requirements. We help innovators successfully enter and expand into new markets and accelerate revenue.
Services and Expertise
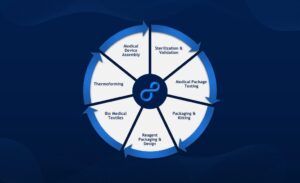
Start up. Speed up. Scale up. Founded in 1997, Life Sciences Outsourcing was born of a need to enhance and advance medical innovation. We are an FDA-registered and ISO 13485-certified organization with services and capabilities spanning the entire medical device product life-cycle – from turnkey manufacturing, testing, validation, and sterilization to precision packaging, fulfillment, and distribution.
Each service is purpose-built to deliver proven solutions at the most critical points in the product development process, reducing costs and accelerating time to market.

One-Stop Shop
Agile and Flexible
We act as an external extension of client teams with the depth and expertise to guide, support, and adapt to evolving needs at every turn. With onboarding in as little as one week, our work with clients is rooted in collaboration and shared objectives.


Regulatory Expertise

News & Info

Life Science Outsourcing Sets a New Standard in Medical Device Manufacturing with NIST 800-171 Compliance

What do Medical Device OEMs Need to Know for MDR CE Mark Recertification in Europe?