Accelerated & Real-Time Aging
Life Science Outsourcing has proven expertise and industry qualifications in medical device packaging design and validation. We work closely with your team to implement effective primary and secondary package design solutions and ASTM F1980 outsourcing to deliver your device to market faster.
Are you looking for guidance for your medical package age testing? This accelerated aging calculator generates a table of values for calculations based upon ASTM F1980, otherwise known as “Accelerated Aging of Sterile Medical Device Packages.”
Accelerated Aging Calculator
This calculator generates a table of values for calculations based upon ASTM F1980, Accelerated Aging of Sterile Medical Device Packages.
Successful Medical Device Package Design
Our primary objective is to ensure your medical device arrives safely and intact to its final destination. Our team has experience designing a variety of custom medical device packages, including low-volume plastic packaging and low-cost tooling, as well as high-volume plastic packaging.
We partner with clients to develop individual or pre-validated packaging recommendations and configurations.Durability testing and integrity verification is completed prior to making any final determinations on tooling and production quantities. We will then provide you with documentary evidence of compliance.
Our in-house packaging validation capabilities are aligned with the industry’s most comprehensive regulatory requirement, ISO 11607, “Packaging for Terminally Sterilized Medical Devices.”
Medical Primary Packaging: Product Packaging
Primary packaging is the first level of medical product packaging containing the actual product. A poor fit between the product and the packaging will often result in damage, the cost of which usually exceeds any cost savings gained by using less-than-ideal packaging. The best way to determine the style and features necessary to protect and enhance your product is to work with an experienced packaging engineer or carton designer. Life Science Outsourcing offers both for your benefit.
Pouch Styles
Chevron Peel Pouch
These are most commonly used for sterile medical products that don’t require the rigidity or other protective characteristics of a thermoformed tray. The most popular materials used in this pouch construction are a Tyvek back and a clear film face.
Corner Peel Pouch
Corner peel pouches use the same construction as a chevron pouch with a different seal configuration. They are used when it is ideal for the product to fit close to the top seal of the pouch or for bulky products.
Squared Sealed (No-Peel, Tear) Pouch
This style of pouch is commonly used for multi-layer laminate barrier materials. The heat seal is permanent and cannot be peeled apart. Therefore, a tear notch is typically provided in the heat seal near the top of the pouch to facilitate opening.
Standard Method of Dimensioning Pouches
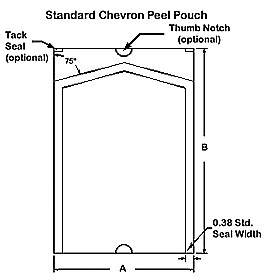
- All pouches, regardless of style, are defined by their outside dimensions
- The “A” dimension is the opening or width and is always listed first.
- The “B” dimension is the length and is always listed second.
- Example: 4″ x 8″. The 4″ dimension is the width and the 8″ dimension is the length of the pouch.
Uncoated Tyvek to Film | Coated Tyvek to Film | Uncoated Paper to Film | Coated Paper to Film | Film to Film | Laminate to Laminate | |
|---|---|---|---|---|---|---|
Chevron Peel Pouch | ||||||
Corner Peel Pouch | ||||||
Square Peel Pouch | ||||||
Header Pouch
| ||||||
Material Properties | 1, 2. 3, 6,7, & 11 | 1, 2, 5, 7 & 11
| 1, 2, 3, 4, 6, 7 & 11
| 1, 2, 5, 7 & 11
| 2, 5, 8, & 10
| 2, 3, 5, 9 & 12
|
Medical Secondary Packaging: Barrier Protection
Secondary packaging, or protective packaging, protects the primary sterile barrier and the medical device contents from damage.
Folding Cartons
Folding cartons provide excellent secondary protection for individually packaged sterile products as well as multiple unit packs. For multiple unit packs, the carton can be designed as a dispenser carton by including a perforated area into the carton.
There are many styles of folding cartons and numerous specialized features can be added to these design styles. Although some simple styles can be ordered off the shelf, most folding cartons used for medical products are custom-designed to fit the product and to incorporate the specialized features which enhance product presentation. A poor fit between the carton and the inner package/product may result in product damage, which can become more costly than using less-than-ideal off-the-shelf cartons.
Shipping Containers
The shipping container is a corrugated box with sufficient strength for shipping, sterilization, and storage of medical devices or any other medical product.
Corrugated fiberboard is made up of two separate components: the linerboard and the medium. The linerboard is the flat facing and the medium is the fluted paper glued between the liners.
Flute profiles: There are many flute profiles used in the construction of corrugated board. The arched flutes create a very rigid and strong medium. The flutes also act as a cushion against minor impact. The most common configurations include B-Flute, C-Flute, E-Flute, and Double Wall constructions. Type of corrugation used in construction of a shipping container are determined by the physical size, weight, and fragility of the product(s) going inside the box.
Burst strength: This type of flute is only part of the equation in determining the strength and durability of the container. Since different grades of material can be used for both the linerboard and the medium, burst strength ratings are also important. Simply put, burst strength is a standardized test method used by all corrugated manufacturers to rate the penetration resistance of the container wall. The higher the rating, the stronger the box. A 200-pound test is commonly used but may not be adequate for delicate products or heavy products.
Shipping container style: The most widely used style of shipping container is the regular slotted container (RSC). The RSC can be used in conjunction with inner packing materials, including corrugated inserts, bubble wrap, and rigid or soft foam inserts to further protect the product.
Shipping containers and folding cartons are defined first by style; second by the inside dimensions length x width x depth; third by the material (flute style and pound test for corrugated) and finally if the carton is printed or plain.
Documentation Support
Developing manufacturing documentation and a quality system that is compliant, efficient, and easy to maintain as you grow can be a burdensome effort. If done improperly, it can lead to significant consequences and challenges.
Partnering with the Life Science Outsourcing team provides access to manufacturing documentation and a quality system that has been audited by the FDA and is reviewed and audited over 50 times a year by our customers.
Because we are building from this extensively audited quality system, the manufacturing documentation that we develop is efficient and easy to use while exceeding industry and regulatory standards. Our quality and process engineers can build customized manufacturing documentation that meets your specific needs and allows your company to tap into existing documentation and processes.
Manufacturing Documentation Services:
- Development of customized documentation
- Process development
- Implementation and adoption into practice
- Training of personnel – including ISO 13485, FDA QSR (GMPs), Corrective Actions
- Continuous Improvement/Gap Assessments – including ISO 13485, FDA QSR (GMPs)
Front-end Design Assistance
Life Science Outsourcing’s product and package design and engineering support services provide guidance on design, details, and development. This includes comprehensive consultation services.
- Design for manufacturability analysis
- Consult on parts design
- Consult on material selection
- Sterile package design
- Shelf and shipping box design and configuration
Shelf and Shipping Box Design and Configuration
Through our extensive work with startups, Life Science Outsourcing’s engineering teams have regularly manufactured products for customer clinical trials. Our experts provide label designing, tooling and fixture designing, risk analysis, failure mode effect analysis (FMEA), product inspections, and process validations.
Through our comprehensive suite of solutions, Life Science Outsourcing coordinates support services such as sterilization validation and package testing. In addition, our engineers can assist you in defining and developing assembly processes, writing product and packaging specifications, and creating validation protocols and reports.
Contact Us To Start Your Project
Please provide your details below, and one of our experienced sales representatives will get back to you shortly. Let’s take the first step towards a successful partnership.
Job Seeker? This form is for customer inquiries only. Job applications submitted here will not be considered.




