As the Director of Regulatory and Quality Assurance at Life Science Outsourcing’s East Coast location, Toni McBride leads the development, management, implementation and sustainability of our ISO 13485:2016 Quality Management System, and ensures the effective implementation of those standards across the entire plant.
McBride also directs the effective implementation and execution of all quality assurance and improvement activities in compliance with LSO’s FDA registration under 21CFR Part 820, including the recent implementation of LSO’s comprehensive electronic quality management system (eQMS) which linked all of LSO’s critical-to-quality functions though a comprehensive cloud-based control system.
Additionally, McBride is responsible for overseeing the regulatory compliance program and safety and risk management programs across the entire plant.
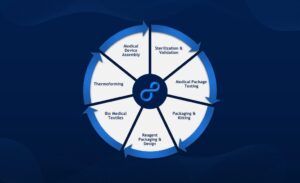
Most recently, McBride spearheaded the qualification and validation of LSO East’s in-house ethylene oxide (EtO) sterilization system, providing our customers a validated turnkey packaging solution from packaging design through sterilization and release
Toni McBride has over 25 years of operational and quality assurance expertise in the medical device industry, ranging from Manufacturing Manager to her current Director role with Life Science Outsourcing. Her comprehensive, hands-on expertise with the entire value stream creates a top-down total employee engagement with the quality management system and ensures that LSO delivers exceptional products and services to our customers and their patients, each and every time.